| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
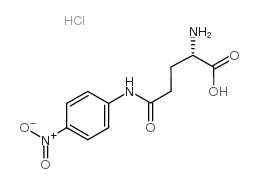 |
L-谷氨酸γ-(p-硝基苯胺)盐酸盐
CAS:67953-08-6 |
|
 |
L-γ-谷氨酰对硝基苯胺一水合物
CAS:7300-59-6 |