| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
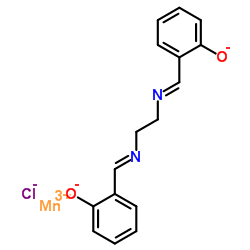 |
N,N'-双(亚水杨基)-1,2-乙二胺氯化-锰(III)
CAS:53177-12-1 |
|
 |
黄钟花醌
CAS:84-79-7 |