A family 2 pectate lyase displays a rare fold and transition metal-assisted beta-elimination.
D Wade Abbott, Alisdair B Boraston
文献索引:J. Biol. Chem. 282(48) , 35328-36, (2007)
全文:HTML全文
摘要
The family 2 pectate lyase from Yersinia enterocolitica (YePL2A), solved to 1.5A, reveals it to be the first prokaryotic protein reported to display the rare (alpha/alpha)(7) barrel fold. In addition to its apo form, we have also determined the structure of a metal-bound form of YePL2A (to 2.0A) and a trigalacturonic acid-bound substrate complex (to 2.1A) Although its fold is rare, the catalytic center of YePL2A can be superimposed with structurally unrelated families, underlining the conserved catalytic amino acid architecture of the beta-elimination mechanism. In addition to its overall structure, YePL2A also has two other unique features: 1) it utilizes a metal atom other than calcium for catalysis, and 2) its Brønstead base is in an alternate conformation and directly interacts with the uronate group of the substrate.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
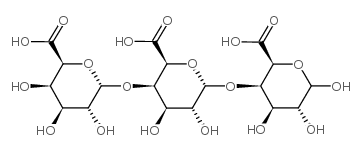 |
三半乳糖醛酸
CAS:6037-45-2 |
C18H26O19 |
|
Molecular characterization of a thermophilic endo-polygalact...
2014-12-31 [J. Agric. Food Chem. 62(52) , 12686-94, (2015)] |
|
Oligosaccharide formation during commercial pear juice proce...
2016-08-01 [Food Chem. 204 , 84-93, (2016)] |
|
A Novel Acid-Stable Endo-Polygalacturonase from Penicillium ...
2016-06-28 [J. Microbiol. Biotechnol. 26 , 989-98, (2016)] |
|
Purification and Properties of Polygalacturonase Produced by...
2013-01-01 [Enzyme Res. 2013 , 438645, (2013)] |
|
Horizontal Gene Transfer of Pectinases from Bacteria Precede...
2016-01-01 [Sci. Rep. 6 , 26388, (2016)] |