Design, synthesis, antimicrobial evaluation and molecular docking studies of some new thiophene, pyrazole and pyridone derivatives bearing sulfisoxazole moiety.
Tamer Nasr, Samir Bondock, Sameh Eid
文献索引:Eur. J. Med. Chem. 84 , 491-504, (2014)
全文:HTML全文
摘要
Development of new antimicrobial agents is a good solution to overcome drug-resistance problems. In this context, new functionalized thiophene, acrylamide, arylhydrazone, pyrazole and pyridone derivatives bearing sulfisoxazole moiety were designed, synthesized and evaluated for their in vitro antibacterial and antifungal activities. Among the synthesized compounds, thiophene 4d and 6-thioglucosylpyridone 17 displayed significant antibacterial activities against Escherichia coli (MIC, 0.007 μg/mL vs gentamycin 1.95 μg/mL) and Bacillis subtilis (MIC, 0.007 μg/mL vs ampicillin 0.24 μg/mL), respectively. Whereas, the pyrazole 6 showed the highest antifungal activity against Aspergillus fumigates (MIC, 0.03 μg/mL vs amphotericin B 0.12 μg/mL). In general, most of the synthesized compounds exhibited better antimicrobial activities than sulfisoxazole; this might be attributed to the synergistic effect of the sulfonamide and attached heterocyclic moieties as well as the increased lipophilic characters of the synthesized compounds. Molecular docking studies indicated that the synthesized compounds could occupy both p-amino benzoic acid (PABA) and pterin binding pockets of the dihydropteroate synthase (DHPS), suggesting that the target compounds could act by the inhibition of microbial DHPS enzyme. The results provide important information for the future design of more potent antimicrobial agents.Copyright © 2014 Elsevier Masson SAS. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
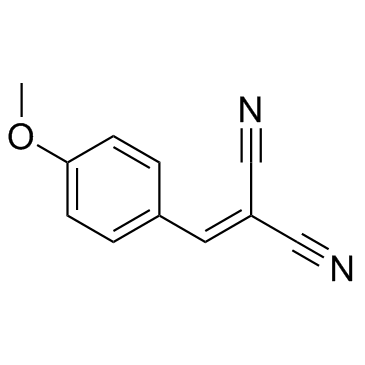 |
酪氨酸磷酸化抑制剂A1
CAS:2826-26-8 |
C11H8N2O |
|
Tyrphostins I: synthesis and biological activity of protein ...
1989-10-01 [J. Med. Chem. 32 , 2344, (1989)] |
|
Blocking of EGF-dependent cell proliferation by EGF receptor...
1988-11-11 [Science 242(4880) , 933-5, (1988)] |
|
Human fibroblast growth factor receptor 1 is a co-receptor f...
1999-01-01 [Nat. Med. 5(1) , 71-7, (1999)] |
|
Cyclic stretch regulates autocrine IGF-I in vascular smooth ...
1999-04-01 [Am. J. Physiol. 276(4 Pt 1) , E697-705, (1999)] |
|
Tyrosine kinase inhibitors block calcium channel currents in...
1992-12-30 [Biochem. Biophys. Res. Commun. 189(3) , 1620-3, (1992)] |