| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
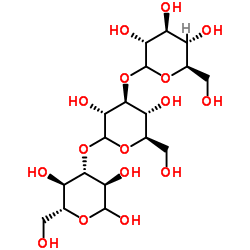 |
昆布多糖
CAS:9008-22-4 |
|
 |
β-葡聚糖酶 来源于黑曲霉
CAS:9074-98-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
昆布多糖
CAS:9008-22-4 |
|
 |
β-葡聚糖酶 来源于黑曲霉
CAS:9074-98-0 |