Gold(I)-catalyzed tandem reactions initiated by hydroamination of alkynyl carbamates: application to the synthesis of nitidine.
Taro Enomoto, Anne-Lise Girard, Yoshizumi Yasui, Yoshiji Takemoto
文献索引:J. Org. Chem. 74(23) , 9158-64, (2009)
全文:HTML全文
摘要
As a convenient and direct synthesis of 1,2-dihydroisoquinolines, the gold(I)-catalyzed intramolecular hydroamination of (2-alkynyl)benzyl carbamates has been developed. The reaction with cationic gold(I) complex [AuCl(PPh(3))/AgNTf(2)] proceeded at room temperature, giving the desired 6-endo adducts. The addition of alcohol efficiently promoted the reaction, and the amount of the catalyst could be reduced to 1 mol %. However, the alkynes bearing either an electron-deficient aryl group or an alkyl group resulted in predominant production of 5-exo adducts. In such cases, use of a bulky gold catalyst, AuCl[(o-biPh)((t)Bu)(2)P]Cl/AgNTf(2), improved the regioselectivity, giving the 6-endo adducts in better yields. Furthermore, the hydroamination of alkynyl carbamates bearing an acetal or enone was successfully applied to the concise synthesis of tetracyclic heterocycles such as nitidine via the single catalyst-mediated tandem cyclization which consists of a condensation or a Michael addition of the resulting enecarbamates.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
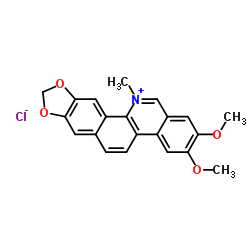 |
氯化两面针碱
CAS:13063-04-2 |
C21H18ClNO4 |
|
Nitidine chloride inhibits hepatocellular carcinoma cell gro...
2013-07-01 [Int. J. Mol. Med. 32(1) , 79-84, (2013)] |
|
Alkaloids, amides and antispasmodic activity of Zanthoxylum ...
2002-06-01 [Planta Med. 68(6) , 534-8, (2002)] |
|
The role of the iminium bond in the inhibition of reverse tr...
1998-11-01 [J. Pharm. Pharmacol. 50(11) , 1307-15, (1998)] |
|
Distinct G-quadruplex structures of human telomeric DNA form...
2010-12-01 [Fitoterapia 81(8) , 1026-32, (2010)] |
|
Development and validation of an LC-ESI-MS/MS method for the...
2012-03-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 887-888 , 43-7, (2012)] |