Correlation between the rate order and the number of molecules in the reaction of trimethyl phosphite with water in acetonitrile solvent.
Shinichi Yamabe, Noriko Tsuchida, Shoko Yamazaki
文献索引:J. Phys. Chem. A 114(43) , 11699-707, (2010)
全文:HTML全文
摘要
Density functional theory calculations of the title reaction, P(OCH₃)₃ + (H₂O)(n) in CH₃CN, were conducted, where n is the number of water molecules. Two routes, the routes suggested by (A) Aksnes and (B) Arbuzov, were traced with various n values. Both routes consist of two transition states (TSs) and one intermediate. Route B was found to be more likely than route A. In the former, the activation free energy (ΔG(‡)) of n = 3 is slightly smaller than that of n = 2. The n = 3 TS geometry is composed of a nucleophile H₂O, a proton donor H₂O, and an auxiliary one. Indeed, the geometry appears to be plausible for ready proton relays along hydrogen bonds, but it is inconsistent with the observed third-order rate constant. Catalytic water molecules were added to the n = 2 and 3 bond-interchange circuits. Then route B with n = 2 + 2 was found to be best. By n = 2 + 10 and n = 3 + 12 models, the n = 2 based route B was confirmed to be likely.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
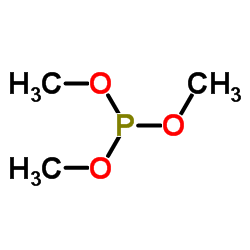 |
亚磷酸三甲酯
CAS:121-45-9 |
C3H9O3P |
|
Interactions of multitargeted kinase inhibitors and nucleosi...
2015-01-01 [J. Chromatogr. A. 1421 , 154-61, (2015)] |
|
Rapid modification of the insect elicitor N-linolenoyl-gluta...
2010-01-01 [BMC Plant Biol. 10 , 164, (2010)] |
|
The functionalisation of saturated hydrocarbons--XXX. Model ...
1994-04-01 [Bioorg. Med. Chem. 2(4) , 259-66, (1994)] |
|
Surface characterization and platelet adhesion studies of pl...
1999-08-01 [Biomaterials 20(16) , 1439-47, (1999)] |
|
Trimethyl phosphite as a trap for alkoxy radicals formed fro...
2003-03-19 [J. Am. Chem. Soc. 125(11) , 3248-59, (2003)] |