Proteolysis of cyclic AMP phosphodiesterase-II attenuates its ability to be inhibited by compounds which exert positive inotropic actions in cardiac tissue.
B Price, N J Pyne, M D Houslay
文献索引:Biochem. Pharmacol. 36(23) , 4047-54, (1987)
全文:HTML全文
摘要
Extraction of frozen canine cardiac muscle rendered soluble over 90% of the cyclic AMP phosphodiesterase activity. The residual activity was membrane-bound. Ion exchange chromatography of the soluble activity on DE-52 allowed for the resolution of three distinct cyclic AMP phosphodiesterase fractions termed PDE-I, PDE-II and PDE-III in order of elution from the column by a linear NaCl gradient. The relative ratio of cyclic AMP phosphodiesterase activity exhibited by these three peaks was 1:0.65:0.82 and of cyclic GMP phosphodiesterase activity was 1:0.52:0.05 for PDE-I, PDE-II and PDE-III respectively. PDE-II and PDE-III were further purified by re-chromatography on DE-52. Fractions PDE-II and PDE-III were thermolabile at 50 degrees, decaying as single exponentials with half lives of 180 sec and 77 sec respectively. All three species exhibited non-linear Lineweaver-Burke plots for the hydrolysis of cyclic AMP, exhibiting both high and low affinity components. Hydrolysis of cyclic GMP by all three components obeyed normal kinetics, yielding linear plots. PDE-I was a Ca2+/calmodulin-activated species which exhibited a low Km for both cyclic AMP and cyclic GMP but hydrolysed cyclic GMP with a higher Vmax than for cyclic AMP. PDE-II exhibited a much lower Km for cyclic AMP than for cyclic GMP and a much higher Vmax for the hydrolysis of cyclic AMP. PDE-III exhibited a low Km for both cyclic AMP and cyclic GMP, however, its Vmax for cyclic AMP was about 40-fold higher than for cyclic GMP. Cyclic GMP acted as a potent inhibitor (IC50 = 6.3 microM) of cyclic AMP hydrolysis catalysed by PDE-III but not of the hydrolysis of cyclic AMP by PDE-II (IC50 = 33.2 microM). The phosphodiesterase inhibitors milrinone, CI-930, UK-35,493, carbazeran and buquineran acted as potent inhibitors of cyclic AMP hydrolysis catalysed by both PDE-II and PDE-III enzymes. They did not inhibit PDE-I activity. PDE-II, when prepared in the absence of protease inhibitors exhibited a reduced potency to inhibition by these compounds. Treatment of purified PDE-II with trypsin caused a reduction in enzyme activity and reduced dramatically the sensitivity of PDE-II activity to inhibition by these various compounds. The action of proteolysis in attenuating the inhibitory effect of these compounds on PDE-II was most dramatic with CI-930, milrinone, amrinone, buquineran and UK35,493 and least dramatic with carbazeran and IBMX.(ABSTRACT TRUNCATED AT 400 WORDS)
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
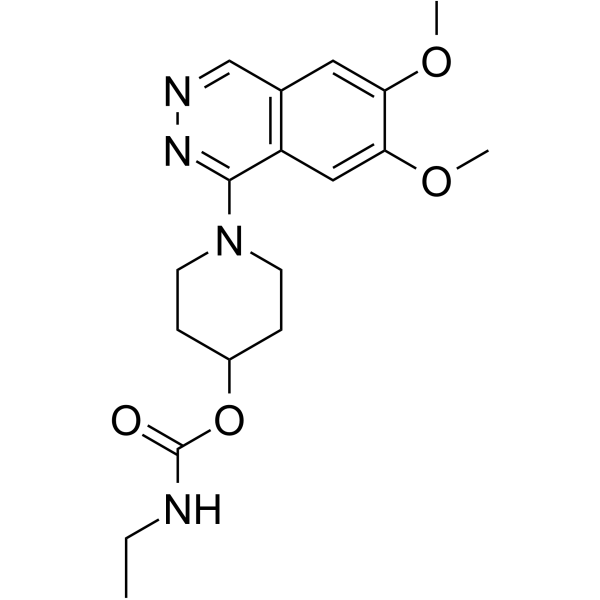 |
卡巴泽伦
CAS:70724-25-3 |
C18H24N4O4 |
|
Aldehyde oxidase activity in fresh human skin.
2014-12-01 [Drug Metab. Dispos. 42(12) , 2049-57, (2014)] |
|
A novel reaction mediated by human aldehyde oxidase: amide h...
2015-06-01 [Drug Metab. Dispos. 43 , 908-15, (2015)] |
|
Chronotropic and inotropic actions of amrinone, carbazeran a...
1989-09-01 [Br. J. Pharmacol. 98 , 291-301, (1999)] |
|
Application of a Micropatterned Cocultured Hepatocyte System...
2016-02-01 [Drug Metab. Dispos. 44 , 172-9, (2016)] |
|
Deuterium isotope effects on drug pharmacokinetics. I. Syste...
2012-03-01 [Drug Metab. Dispos. 40(3) , 625-34, (2012)] |