| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
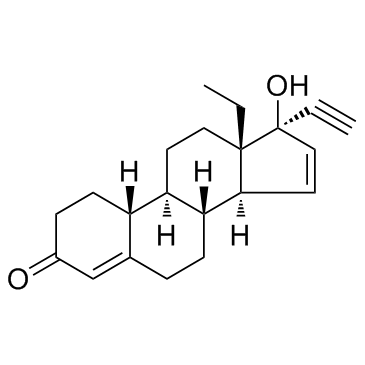 |
孕二烯酮
CAS:60282-87-3 |
|
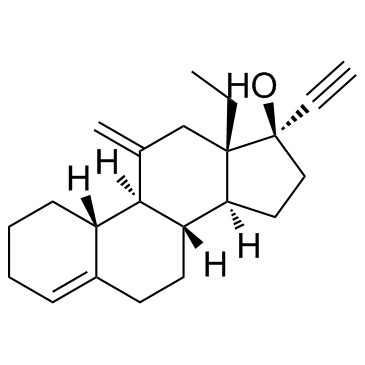 |
去氧孕烯
CAS:54024-22-5 |
|
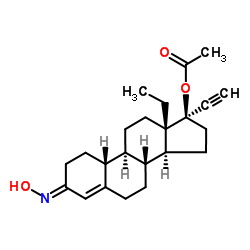 |
炔诺肟酯
CAS:35189-28-7 |