| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
山梨醇脱氢酶(微生物)
CAS:9028-21-1 |
|
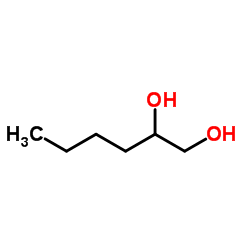 |
1,2-己二醇
CAS:6920-22-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
山梨醇脱氢酶(微生物)
CAS:9028-21-1 |
|
 |
1,2-己二醇
CAS:6920-22-5 |