Cyclic poly(alpha-peptoid)s and their block copolymers from N-heterocyclic carbene-mediated ring-opening polymerizations of N-substituted N-carboxylanhydrides.
Li Guo, Donghui Zhang
文献索引:J. Am. Chem. Soc. 131(50) , 18072-4, (2009)
全文:HTML全文
摘要
N-Heterocyclic carbene (NHC)-mediated ring-opening polymerization (ROP) of N-substituted N-carboxylanhydride ((N)R-NCA) yields cyclic poly(alpha-peptoid)s with controlled molecular weights (M(n) = 3-30 kg mol(-1)) and narrow molecular weight distributions (PDI = 1.04-1.12). The reactions exhibit characteristics of a living polymerization with minimal chain transfer. This enables the facile synthesis of cyclic diblock copoly(alpha-peptoid)s through sequential monomer addition. The cyclic polymer architectures were verified by MALDI-TOF mass spectrometry and intrinsic viscosity measurements. Mark-Houwink-Sakurada plot analyses revealed that cyclic poly(alpha-peptoid)s prepared from NHC-mediated polymerizations exhibit lower intrinsic viscosities than their linear analogues prepared from primary amine-initiated polymerizations. The ratio of their intrinsic viscosities is consistent with the former being mostly cyclic.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
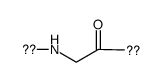 |
聚甘氨酸
CAS:25718-94-9 |
C2H3NO |
|
Aggregation of capped hexaglycine strands into hydrogen-bond...
2011-02-17 [J. Phys. Chem. B 115 , 1562-1570, (2011)] |
|
Catalytic oxidation of uric acid at the polyglycine chemical...
1997-08-01 [Analyst 122 , 839-841, (1997)] |
|
One-pot electrosynthesis of polyglycine-like thin film on pl...
2010-06-30 [Talanta 82 , 417-421, (2010)] |
|
Communications: Is quantum chemical treatment of biopolymers...
2010-06-21 [J. Chem. Phys. 132(23) , 231101, (2010)] |
|
Structure of sodiated octa-glycine: IRMPD spectroscopy and m...
2010-05-01 [J. Am. Soc. Mass Spectrom. 21(5) , 728-38, (2010)] |