Bioscience, Biotechnology, and Biochemistry
2007-06-01
A concise synthetic approach to beta,gamma-dehydrocurvularin: synthesis of (+/-)-di-O-methyl-beta,gamma-dehydrocurvularin.
Takuho Miyagi, Shigefumi Kuwahara
文献索引:Biosci. Biotechnol. Biochem. 71(6) , 1592-4, (2007)
全文:HTML全文
摘要
A concise synthesis of di-O-methyl-beta,gamma-dehydrocurvularin, the di-O-methylated derivative of the naturally occurring nematicidal macrolide, beta,gamma-dehydrocurvuralin, was accomplished by starting from a commercially available aromatic carboxylic acid in a three-step sequence consisting of esterification, Friedel-Crafts acylation, and microwave-promoted ring-closing metathesis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
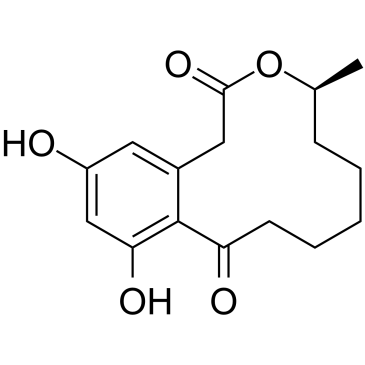 |
弯孢霉菌素
CAS:10140-70-2 |
C16H20O5 |
相关文献:
更多...
|
Sulfur-containing cytotoxic curvularin macrolides from Penic...
2013-11-22 [J. Nat. Prod. 76(11) , 2145-9, (2013)] |
|
Metabolite production by differentUlocladiumspecies
2008-01-01 [Int. J. Food Microbiol. 126(1-2) , 172-9, (2008)] |
|
[Molecular basis of probes for cytoskeletal proteins].
1993-08-01 [Tanpakushitsu Kakusan Koso. 38(11) , 1730-41, (1993)] |
|
[Functional and distributional changes of MTOC's in centroso...
1989-09-01 [Tanpakushitsu Kakusan Koso. 34(12 Suppl) , 1610-7, (1989)] |
|
[Biological activity of Penicillium sp. 10-51 exometabolites...
2012-01-01 [Mikrobiol. Z. 74(4) , 52-6, (2012)] |