Total synthesis of (s)-(+)-citreofuran by ring closing alkyne metathesis.
Alois Fürstner, Anne-Sophie Castanet, Karin Radkowski, Christian W Lehmann
文献索引:J. Org. Chem. 68(4) , 1521-8, (2003)
全文:HTML全文
摘要
A concise total synthesis of citreofuran 4 is described, a structurally unique octaketide derivative belonging to the curvularin family. Key steps involve the elaboration of orsellinic acid methyl ester 5 to acid 14, which converts, on attempted formation of the corresponding acid chloride, to the 3-alkoxyisocoumarin derivative 20. This heterocycle can be used as an activated ester to give ketone 21 on treatment with 3-pentynylmagnesium bromide in the presence of TMSCl as the activating agent. Ring- closing alkyne metathesis (RCAM) of diyne 21 catalyzed by (tBuO)(3)W[triple bond]CCMe(3) affords the strained cycloalkyne 22. Treatment with acid renders its triple bond susceptible to nucleophilic attack by the adjacent carbonyl group, thus leading to a transannular cycloaromatization with formation of the intact skeleton of citreofuran. An X-ray crystallographic study reveals conformational details about this natural product. Finally, it is shown that 4 as well as its protected precursor 23 are able to cleave double-stranded DNA under oxidative conditions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
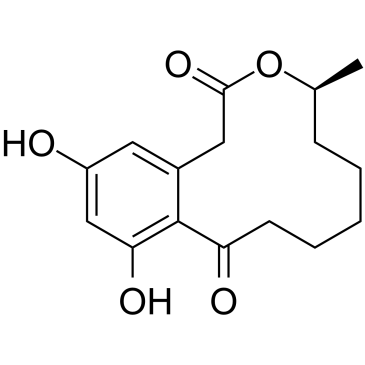 |
弯孢霉菌素
CAS:10140-70-2 |
C16H20O5 |
|
Sulfur-containing cytotoxic curvularin macrolides from Penic...
2013-11-22 [J. Nat. Prod. 76(11) , 2145-9, (2013)] |
|
Metabolite production by differentUlocladiumspecies
2008-01-01 [Int. J. Food Microbiol. 126(1-2) , 172-9, (2008)] |
|
[Molecular basis of probes for cytoskeletal proteins].
1993-08-01 [Tanpakushitsu Kakusan Koso. 38(11) , 1730-41, (1993)] |
|
[Functional and distributional changes of MTOC's in centroso...
1989-09-01 [Tanpakushitsu Kakusan Koso. 34(12 Suppl) , 1610-7, (1989)] |
|
[Biological activity of Penicillium sp. 10-51 exometabolites...
2012-01-01 [Mikrobiol. Z. 74(4) , 52-6, (2012)] |