ELIXYS - a fully automated, three-reactor high-pressure radiosynthesizer for development and routine production of diverse PET tracers.
Mark Lazari, Kevin M Quinn, Shane B Claggett, Jeffrey Collins, Gaurav J Shah, Henry E Herman, Brandon Maraglia, Michael E Phelps, Melissa D Moore, R Michael van Dam
文献索引:EJNMMI Res. 3 , 52, (2013)
全文:HTML全文
摘要
Automated radiosynthesizers are vital for routine production of positron-emission tomography tracers to minimize radiation exposure to operators and to ensure reproducible synthesis yields. The recent trend in the synthesizer industry towards the use of disposable kits aims to simplify setup and operation for the user, but often introduces several limitations related to temperature and chemical compatibility, thus requiring reoptimization of protocols developed on non-cassette-based systems. Radiochemists would benefit from a single hybrid system that provides tremendous flexibility for development and optimization of reaction conditions while also providing a pathway to simple, cassette-based production of diverse tracers.We have designed, built, and tested an automated three-reactor radiosynthesizer (ELIXYS) to provide a flexible radiosynthesis platform suitable for both tracer development and routine production. The synthesizer is capable of performing high-pressure and high-temperature reactions by eliminating permanent tubing and valve connections to the reaction vessel. Each of the three movable reactors can seal against different locations on disposable cassettes to carry out different functions such as sealed reactions, evaporations, and reagent addition. A reagent and gas handling robot moves sealed reagent vials from storage locations in the cassette to addition positions and also dynamically provides vacuum and inert gas to ports on the cassette. The software integrates these automated features into chemistry unit operations (e.g., React, Evaporate, Add) to intuitively create synthesis protocols. 2-Deoxy-2-[18F]fluoro-5-methyl-β-l-arabinofuranosyluracil (l-[18F]FMAU) and 2-deoxy-2-[18F]fluoro-β-d-arabinofuranosylcytosine (d-[18F]FAC) were synthesized to validate the system.l-[18F]FMAU and d-[18F]FAC were successfully synthesized in 165 and 170 min, respectively, with decay-corrected radiochemical yields of 46% ± 1% (n = 6) and 31% ± 5% (n = 6), respectively. The yield, repeatability, and synthesis time are comparable to, or better than, other reports. d-[18F]FAC produced by ELIXYS and another manually operated apparatus exhibited similar biodistribution in wild-type mice.The ELIXYS automated radiosynthesizer is capable of performing radiosyntheses requiring demanding conditions: up to three reaction vessels, high temperatures, high pressures, and sensitive reagents. Such flexibility facilitates tracer development and the ability to synthesize multiple tracers on the same system without customization or replumbing. The disposable cassette approach simplifies the transition from development to production.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
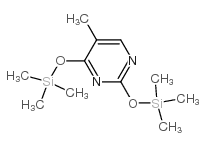 |
O,O'-双(三甲基甲硅烷基)胸腺嘧啶
CAS:7288-28-0 |
C11H22N2O2Si2 |
|
A NEW SYNTHETIC METHOD OF NUCLEOSIDES.
1963-11-01 [Chem. Pharm. Bull. 11 , 1470, (1963)] |
|
Studies on synthetic nucleosides. I. Trimethylsilyl derivati...
1964-03-01 [Chem. Pharm. Bull. 12 , 352, (1964)] |
|
A general synthesis of pyrimidine nucleosides.
1970-06-01 [Angew. Chem. Int. Ed. Engl. 9 , 461, (1970)] |
|
[Angew. Chem. Int. Ed. Engl. 8 , 976,, (1969)] |