| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
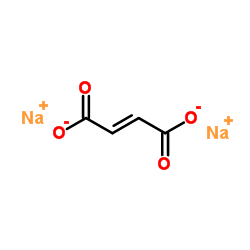 |
富马酸钠
CAS:17013-01-3 |
|
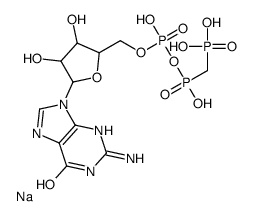 |
GppCp(GMPPCP)
CAS:10470-57-2 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
富马酸钠
CAS:17013-01-3 |
|
 |
GppCp(GMPPCP)
CAS:10470-57-2 |