Synthesis and anti-HIV activity of thiocholesteryl-coupled phosphodiester antisense oligonucleotides incorporated into immunoliposomes.
O Zelphati, E Wagner, L Leserman
文献索引:Antiviral Res. 25(1) , 13-25, (1994)
全文:HTML全文
摘要
Encapsulation of oligonucleotides in antibody-targeted liposomes (immunoliposomes) which bind to target cells permits intracellular delivery of the oligonucleotides. This approach circumvents problems of extracellular degradation by nucleases and poor membrane permeability which free phosphodiester oligonucleotides are subject to, but leaves unresolved the inefficiency of encapsulation of oligonucleotides in liposomes. We have coupled oligonucleotides to cholesterol via a reversible disulfide bond. This modification of oligonucleotides improved their association with immunoliposomes by a factor of about 10 in comparison to unmodified oligonucleotides. The presence of cholesteryl-modified oligonucleotides incorporated in the bilayer of liposomes did not interfere with the coupling of the targeting protein to the liposome surface. Free or cholesterol coupled oligonucleotides associated with liposomes and directed against the tat gene of HIV-1 were tested for inhibition of HIV-1 proliferation in acutely infected cells. We demonstrate that the cholesteryl-modified as well as unmodified oligonucleotides acquire the target specificity of the antibody on the liposome. Their antiviral activity when delivered into cells is sequence-specific. The activity of these modified or unmodified oligonucleotides to inhibit the replication of HIV was the same on an equimolar basis (EC50 around 0.1 microM). Cholesterol coupled oligonucleotides thus offer increased liposome association without loss of antiviral activity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
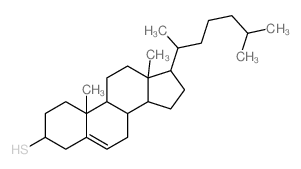 |
巯基胆固醇
CAS:1249-81-6 |
C27H46S |
|
The hedgehog receptor patched is involved in cholesterol tra...
2011-01-01 [PLoS ONE 6 , e23834, (2011)] |
|
Poly(amidoamine) conjugates with disulfide-linked cholestero...
2008-10-01 [Biomacromolecules 9(10) , 2693-704, (2008)] |
|
Effect of the nature of the spacer on gene transfer efficaci...
2008-04-24 [J. Med. Chem. 51(8) , 2533-40, (2008)] |
|
Thiocholesterol-based lipids for ordered assembly of bioresp...
2005-03-01 [Mol. Ther. 11(3) , 409-17, (2005)] |
|
Enhanced hepatic uptake and bioactivity of type alpha1(I) co...
2006-05-01 [J. Pharmacol. Exp. Ther. 317(2) , 797-805, (2006)] |