Effective incorporation of 2'-O-methyl-oligoribonucleotides into liposomes and enhanced cell association through modification with thiocholesterol.
B Oberhauser, E Wagner
文献索引:Nucleic Acids Res. 20(3) , 533-8, (1992)
全文:HTML全文
摘要
Cholesterol was linked to 2'-O-methyl-oligoribonucleotides (2'-OMe-RNA) via a disulfide bond by reacting the 3'-(pyridyldithio)-modified 2'-OMe-RNA with thiocholesterol in dichloromethane-methanol solution. This ligation reaction was made possible by a novel strategy in which the highly charged oligonucleotide was rendered soluble in nonaqueous solvent through conversion to a lipophilic amidinium salt. The biodegradable lipophilic modification of 2'-OMe-RNA resulted in a large increase in incorporation of such oligonucleotides into liposomes prepared by reversephase evaporation. Furthermore, association of these modified oligonucleotides with cultured TIB 73 cells was 100-fold higher than that seen with unmodified 2'-OMe-RNA in serum-free medium and about 10 to 30-fold higher in the presence of 10% calf serum. During incubation with cells, release of the internalized oligonucleotide from the thiocholesteryl moiety can be demonstrated.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
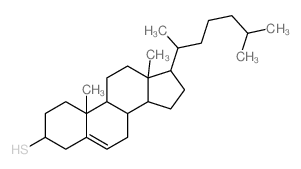 |
巯基胆固醇
CAS:1249-81-6 |
C27H46S |
|
The hedgehog receptor patched is involved in cholesterol tra...
2011-01-01 [PLoS ONE 6 , e23834, (2011)] |
|
Poly(amidoamine) conjugates with disulfide-linked cholestero...
2008-10-01 [Biomacromolecules 9(10) , 2693-704, (2008)] |
|
Effect of the nature of the spacer on gene transfer efficaci...
2008-04-24 [J. Med. Chem. 51(8) , 2533-40, (2008)] |
|
Thiocholesterol-based lipids for ordered assembly of bioresp...
2005-03-01 [Mol. Ther. 11(3) , 409-17, (2005)] |
|
Enhanced hepatic uptake and bioactivity of type alpha1(I) co...
2006-05-01 [J. Pharmacol. Exp. Ther. 317(2) , 797-805, (2006)] |