The 2-aminoethylphosphonate-specific transaminase of the 2-aminoethylphosphonate degradation pathway.
Alexander D Kim, Angela S Baker, Debra Dunaway-Mariano, W W Metcalf, B L Wanner, Brian M Martin
文献索引:J. Bacteriol. 184(15) , 4134-40, (2002)
全文:HTML全文
摘要
The 2-aminoethylphosphonate transaminase (AEPT; the phnW gene product) of the Salmonella enterica serovar Typhimurium 2-aminoethylphosphonate (AEP) degradation pathway catalyzes the reversible reaction of AEP and pyruvate to form phosphonoacetaldehyde (P-Ald) and L-alanine (L-Ala). Here, we describe the purification and characterization of recombinant AEPT. pH rate profiles (log V(m) and log V(m)/K(m) versus pH) revealed a pH optimum of 8.5. At pH 8.5, K(eq) is equal to 0.5 and the k(cat) values of the forward and reverse reactions are 7 and 9 s(-1), respectively. The K(m) for AEP is 1.11 +/- 0.03 mM; for pyruvate it is 0.15 +/- 0.02 mM, for P-Ald it is 0.09 +/- 0.01 mM, and for L-Ala it is 1.4 +/- 0.03 mM. Substrate specificity tests revealed a high degree of discrimination, indicating a singular physiological role for the transaminase in AEP degradation. The 40-kDa subunit of the homodimeric enzyme is homologous to other members of the pyridoxalphosphate-dependent amino acid transaminase superfamily. Catalytic residues conserved within well-characterized members are also conserved within the seven known AEPT sequences. Site-directed mutagenesis demonstrated the importance of three selected residues (Asp168, Lys194, and Arg340) in AEPT catalysis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
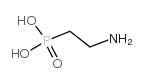 |
2-氨基乙基膦酸
CAS:2041-14-7 |
C2H8NO3P |
|
Phosphonoacetate biosynthesis: in vitro detection of a novel...
2011-01-01 [Mikrobiologiia 80(3) , 329-34, (2011)] |
|
Aminomethylphosphonate and 2-aminoethylphosphonate as (31)P-...
2000-08-01 [NMR Biomed. 13(5) , 289-96, (2000)] |
|
Combined C-H functionalization/O-H insertion reaction to for...
2013-09-07 [Org. Biomol. Chem. 11(33) , 5491-9, (2013)] |
|
Structures of an alanine racemase from Bacillus anthracis (B...
2008-05-01 [Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 64(Pt 5) , 327-33, (2008)] |
|
Properties of phosphoenolpyruvate mutase, the first enzyme i...
2003-06-20 [J. Biol. Chem. 278(25) , 22703-8, (2003)] |