Synthesis of (1-->4)-beta-D-xylo-oligosaccharides of dp 4-10 by a blockwise approach.
K Takeo, Y Ohguchi, R Hasegawa, S Kitamura
文献索引:Carbohydr. Res. 278(2) , 301-13, (1995)
全文:HTML全文
摘要
Dibutyltin oxide-mediated regioselective chloroacetylation of methyl 1-thio-beta-xylobioside, followed by treatment of the product with 4-methylbenzoyl chloride-pyridine, gave methyl 4-O-chloroacetyl-2,3-di-O-(4-methylbenzoyl)-beta-D-xylopyranosyl-(1-->4) -2.3-di - O-(4-methylbenzoyl)-1-thio-beta-D-xylopyranoside (18) in 70% yield. Coupling of 18 with benzyl alcohol afforded the disaccharide benzyl beta-glycoside, which was O-dechloroacetylated to provide methyl 2,3-di-O-(4-methylbenzoyl)-beta-D-xylopyranosyl-(1-->4)-2,3-di-O-(4- methylbenzoyl)-1-thio-beta-D-xylopyranoside (20). A homologous series of (1-->4)-beta-D-xylo-oligosaccharides from the tetra- to the deca-saccharide have been synthesized in a blockwise manner by using 20 as the glycosyl acceptor, 18, methyl 1-thio-beta-xylobioside pentaacetate, and methyl 1-thio-beta-xylotrioside heptaacetate as the glycosyl donors, and a combination of N-iodosuccinimide-silver triflate as the promoter.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
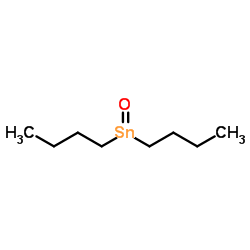 |
二丁基氧化锡
CAS:818-08-6 |
C8H18OSn |
|
Copolyesters made from 1,4-butanediol, sebacic acid, and D-g...
2015-03-09 [Biomacromolecules 16(3) , 868-79, (2015)] |
|
Hydrogen-bond-mediated self-assembly of 26-membered diaza te...
2011-10-21 [J. Org. Chem. 76(20) , 8223-31, (2011)] |
|
Dibutyltin oxide--phenyl isocyanate system for regioselectiv...
1980-01-01 [Nucleic Acids Symp. Ser. (8) , s7-8, (1980)] |
|
[Preparation and spectroscopic studies of diorganotin(IV) co...
2000-01-01 [Acta Pharm. Hung. 70(3-6) , 119-30, (2000)] |
|
Synthesis of 2- and 4-nitrophenyl beta-glycosides of beta-(1...
1995-11-22 [Carbohydr. Res. 277(2) , 231-44, (1995)] |