| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
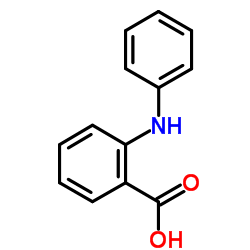 |
N-苯基邻氨基苯甲酸(钒试剂)
CAS:91-40-7 |
|
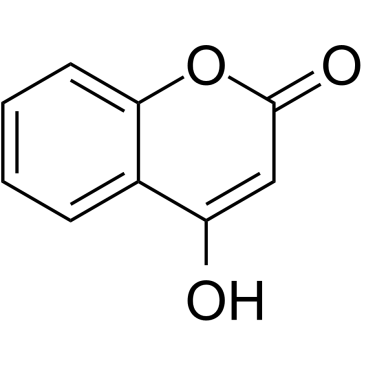 |
4-羟基香豆素; 4-羟基-1-苯并吡喃-2-酮
CAS:1076-38-6 |