In silico screening, structure-activity relationship, and biologic evaluation of selective pteridine reductase inhibitors targeting visceral leishmaniasis.
Jaspreet Kaur, Pranav Kumar, Sargam Tyagi, Richa Pathak, Sanjay Batra, Prashant Singh, Neeloo Singh
文献索引:Antimicrob. Agents Chemother. 55(2) , 659-66, (2011)
全文:HTML全文
摘要
In this study we utilized the concept of rational drug design to identify novel compounds with optimal selectivity, efficacy and safety, which would bind to the target enzyme pteridine reductase 1 (PTR1) in Leishmania parasites. Twelve compounds afforded from Baylis-Hillman chemistry were docked by using the QUANTUM program into the active site of Leishmania donovani PTR1 homology model. The biological activity for these compounds was estimated in green fluorescent protein-transfected L. donovani promastigotes, and the most potential analogue was further investigated in intracellular amastigotes. Structure-activity relationship based on homology model drawn on our recombinant enzyme was substantiated by recombinant enzyme inhibition assay and growth of the cell culture. Flow cytometry results indicated that 7-(4-chlorobenzyl)-3-methyl-4-(4-trifluoromethyl-phenyl)-3,4,6,7,8,9-hexahydro-pyrimido[1,2-a]pyrimidin-2-one (compound 7) was 10 times more active on L. donovani amastigotes (50% inhibitory concentration [IC(50)] = 3 μM) than on promastigotes (IC(50) = 29 μM). Compound 7 exhibited a K(i) value of 0.72 μM in a recombinant enzyme inhibition assay. We discovered that novel pyrimido[1,2-a]pyrimidin-2-one systems generated from the allyl amines afforded from the Baylis-Hillman acetates could have potential as a valuable pharmacological tool against the neglected disease visceral leishmaniasis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
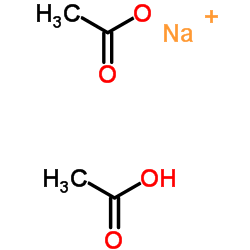 |
双乙酸钠
CAS:126-96-5 |
C4H7NaO4 |
|
Glycaemic index of some commercial gluten-free foods.
2015-09-01 [Eur. J. Nutr. 54 , 1021-6, (2015)] |
|
Sodium Acetate Coated Tenofovir-Loaded Chitosan Nanoparticle...
2016-02-01 [Pharm. Res. 33 , 367-83, (2016)] |
|
Molecular docking, structure-activity relationship and biolo...
2010-08-01 [J. Antimicrob. Chemother. 65(8) , 1742-8, (2010)] |
|
Radioactive in situ hybridization for detecting diverse gene...
2012-01-01 [J. Vis. Exp. (62) , doi:10.3791/3764, (2012)] |
|
[Vertigo and dizziness: the neurologist's perspective].
2013-01-01 [Ophthalmologe 110(1) , 7-15, (2013)] |