Interaction of some trialkyl phosphorothiolates with acetylcholinesterase. Characterization of inhibition, aging and reactivation.
B Clothier, M K Johnson, E Reiner
文献索引:Biochim. Biophys. Acta 660(2) , 306-16, (1981)
全文:HTML全文
摘要
The reaction of bovine erythrocyte acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) with a set of structurally related phosphorothiolates was studied in order to investigate the properties of the phosphorylated enzymes and to identify the leaving group. OOS- and OOS-trimethyl phosphorothiolates and their triethyl analogues inhibit acetylcholinesterase reversibly and by progressive inhibition, and the phosphorylated enzymes undergo both spontaneous reactivation and aging. For each compound the enzyme-inhibitor dissociation constant, and the rate constants for inhibition (ka), reactivation and aging have been derived. The OOS-compounds are more potent inhibitors than the OOS-compounds, and the derived inhibited enzymes reactivate and age faster. By comparing reactivation and aging rate constants with those obtained from phosphorylated enzymes of known structure it was concluded that the leaving group of during phosphorylation is the S-alkyl. SSS-trimethyl and -triethyl phosphorothiolates also form reversible complexes and inhibit the enzyme progressively. With these inhibitors the phosphorylated enzymes did not reactivate either spontaneously or in response to oximes under conditions successful for the other inhibitors. The ka values (37 degrees C, pH 7.4) range from 30 M-1 X min-1 (OOS-trimethyl phosphorothiolate) to 6.7 X 10(3) M-1 X min-1 (OOS-triethyl phosphorothiolate) as compared to 1.25 X 10(5) M-1 X min-1 determined for isomalathion (O, S-dimethyl S-(1,2-dicarbethoxyethyl)-phoshporodithioate), which was used as one of the reference compounds. If the inhibitory potency of the trialkyl phosphorothiolates is calculated from measurements made after a fixed preincubation time the results in ka values will be misleading.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
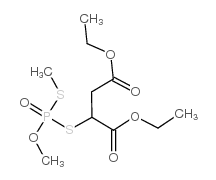 |
异甲基硫
CAS:3344-12-5 |
C10H19O6PS2 |
|
Probing the chiral separation mechanism and the absolute con...
2013-03-15 [J. Chromatogr. A. 1281 , 26-31, (2013)] |
|
Comparison of photocatalysis and photolysis of malathion, is...
2007-11-01 [Water Res. 41(19) , 4504-14, (2007)] |
|
An enzyme test for determining isomalathion impurities in wa...
1986-01-01 [Bull. World Health Organ. 64(3) , 397-401, (1986)] |
|
Toxicological properties of trialkyl phosphorothioate and di...
1983-01-01 [J. Environ. Sci. Health B 18(1) , 89-117, (1983)] |
|
Malathion detoxification by human hepatic carboxylesterases ...
2005-01-01 [J. Biochem. Mol. Toxicol. 19(6) , 406-14, (2005)] |