Identification of the metabolites of benzo[f]quinoline and benzo[h]quinoline formed by rat liver homogenate.
E J LaVoie, E A Adams, D Hoffmann
文献索引:Carcinogenesis 4(9) , 1133-8, (1983)
全文:HTML全文
摘要
Benzo[f]quinoline and benzo[h]quinoline are widespread environmental pollutants which have been found to be mutagenic. The metabolism of benzo[f]quinoline and benzo[h]quinoline was investigated using a liver homogenate from Aroclor-pretreated rats. The metabolites of benzo[f]quinoline which were identified were 7,8-dihydroxy-7,8-dihydrobenzo[f]quinoline, 9,10-dihydroxy-9,10-dihydrobenzo[f]quinoline, 7-hydroxybenzo[f]quinoline, and benzo[f]quinoline-N-oxide. Metabolism studies on benzo[f]quinoline performed in the presence of the epoxide hydratase inhibitor, 3,3,3-trichloropropylene oxide, demonstrated that the formation of both of these dihydrodiols can be inhibited. The major metabolites of benzo[h]quinoline were identified as 5,6-dihydroxy-5,6-dihydrobenzo[h]quinoline and 7,8-dihydroxy-7,8-dihydrobenzo[h]quinoline. Benzo[h]quinoline-N-oxide was not detected as a metabolite. In the presence of an epoxide hydratase inhibitor, the major metabolites of benzo[h]quinoline were 5,6-epoxybenzo[h]quinoline and 7-hydroxybenzo[h]quinoline. The difference in the metabolism to N-oxides observed between benzo[h]quinoline and benzo[f]quinoline is consistent with previous observations in which sterically hindered aromatic ring nitrogen compounds such as benzo[h]quinoline are more resistant to N-oxide formation. The nitrogen atom of these aza-arenes with its lone pair of electrons has a significant influence on sites at which dihydrodiols are formed. The data suggest that the aromatic ring nitrogen of these azaphenanthrenes has an effect similar to that of a methyl substituent in directing their metabolic oxidation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
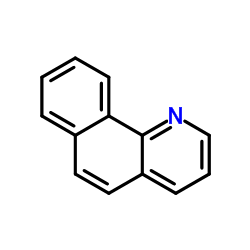 |
7,8-苯并喹啉
CAS:230-27-3 |
C13H9N |
|
Synthesis of novel benzo[h]quinolines: Wound healing, antiba...
2009-01-01 [Eur. J. Med. Chem. 44 , 981-9, (2009)] |
|
Determination of basic nitrogen-containing polynuclear aroma...
2000-05-12 [J. Chromatogr. A. 878(2) , 171-81, (2000)] |
|
Mutagenicity and tumorigenicity of dihydrodiols, diol epoxid...
1989-01-01 [Cancer Res. 49(1) , 20-4, (1989)] |
|
In vivo mutagenicity of benzo[f]quinoline, benzo[h]quinoline...
2004-04-11 [Mutat. Res. 559(1-2) , 83-95, (2004)] |
|
Crowded Cu(I) complexes involving benzo[h]quinoline: pi-stac...
2001-07-02 [Inorg. Chem. 40(14) , 3413-22, (2001)] |