Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration.
Eleonora L Swart, Joost de Jongh, Klaas P Zuideveld, Meindert Danhof, Lambertus G Thijs, Robert J M Strack van Schijndel
文献索引:Am. J. Kidney Dis. 45(2) , 360-71, (2005)
全文:HTML全文
摘要
The objective is to study the population pharmacokinetics of lorazepam and midazolam in critically ill patients with acute renal failure who are treated with continuous venovenous hemofiltration (CVVH).Twenty critically ill patients with acute renal failure on CVVH therapy were administered either lorazepam (n = 10) or midazolam (n = 10) by continuous infusion. CVVH was performed with an ultrafiltrate flow of 2 L/h with filtrate substitution in the predilution or postdilution mode. Blood flow through the 1.9-m 2 cellulose triacetate membrane filter was 180 mL/min. For 48 hours, multiple blood and ultrafiltrate samples were obtained for determination of concentrations of the drug and its metabolites.The pharmacokinetics of lorazepam is described best by a 1-compartment model. No significant covariates were identified. Total-body clearance was 6.4 L/h, and volume of distribution was 376 L. Ultrafiltration clearance was 0.31 L/h, equivalent to approximately 5% of total clearance. Average degree of plasma protein binding was 82.9% for lorazepam, with a sieving coefficient of 0.16 +/- 0.03. For lorazepamglucuronide, degree of plasma protein binding was 39.5%, and sieving coefficient was 0.48 +/- 0.07. The pharmacokinetics of midazolam is described best by a 1-compartment model. No significant covariates were identified. Total-body clearance was 8.5 L/h, and volume of distribution was 157 L. Clearance by ultrafiltration was 0.055 L/h, equivalent to approximately 0.7% of total clearance. Average degree of plasma protein binding was 95.8%, with a sieving coefficient of 0.04 +/- 0.03. For the metabolite 1-hydroxymidazolamglucuronide, average degree of plasma protein binding was 43.4%, with a sieving coefficient of 0.45 +/- 0.06.Neither lorazepam nor midazolam is removed efficiently by CVVH. CVVH contributes significantly to the removal of the glucuronide metabolites lorazepamglucuronide and 1-hydroxymidazolamglucuronide.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
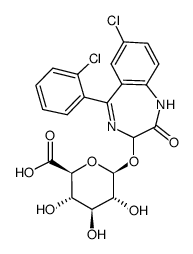 |
Lorazepam glucuronide
CAS:32781-79-6 |
C21H18Cl2N2O8 |
|
Commentary on: Dou C, Bournique J, Zinda M, Gnezda M, Nally ...
2002-03-01 [J. Forensic Sci. 47(2) , 427-8, (2002)] |
|
Biotransformation and excretion of lorazepam in patients wit...
1976-12-01 [Br. J. Clin. Pharmacol. 3(6) , 1033-9, (1976)] |
|
Analysis of lorazepam and its 30-glucuronide in human urine ...
2006-01-01 [J. Sep. Sci. 29(1) , 153-63, (2006)] |
|
Quantitative assay of lorazepam and its metabolite glucuroni...
2006-02-13 [J. Pharm. Biomed. Anal. 40(2) , 389-96, (2006)] |
|
Effect of renal impairment and hemodialysis on lorazepam kin...
1984-05-01 [Clin. Pharmacol. Ther. 35(5) , 646-52, (1984)] |