Optical spectra of lactoperoxidase as a function of solvent.
B Zelent, T Yano, P-I Ohlsson, M L Smith, J Paul, J M Vanderkooi
文献索引:Biochemistry 44(48) , 15953-9, (2005)
全文:HTML全文
摘要
The iron of lactoperoxidase is predominantly high-spin at ambient temperature. Optical spectra of lactoperoxidase indicate that the iron changes from high-spin to low-spin in the temperature range from room temperature to 20 K. The transformation is independent of whether the enzyme is in glycerol/water or solid sugar glass. Addition of the inhibitor benzohydroxamic acid increases the amount of the low-spin form, and again the transformation is independent of whether the protein is in an aqueous solution or a nearly anhydrous sugar. In contrast to lactoperoxidase, horseradish peroxidase remains high-spin over the temperature excursion in both solvents and with addition of benzohydroxamic acid. We conclude that details of the heme pocket of lactoperoxidase allow ligation changes with temperature that are dependent upon the apoprotein but independent of solvent fluctuations. At low pH, lactoperoxidase shows a solvent-dependent transition; the high-spin form is predominant in anhydrous sugar glass, but in the presence of water, the low-spin form is also present in abundance. The active site of lactoperoxidase is not as tightly constrained at low pH as at neutrality, though the enzyme is active over a wide pH range.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
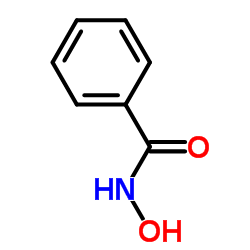 |
苯甲羟肟酸
CAS:495-18-1 |
C7H7NO2 |
|
Multi-target spectral moment QSAR versus ANN for antiparasit...
2010-03-15 [Bioorg. Med. Chem. 18 , 2225-31, (2010)] |
|
Intermediate analogue inhibitors of mandelate racemase: N-Hy...
2007-01-01 [Bioorg. Med. Chem. Lett. 17 , 105-8, (2007)] |
|
Dephosphorylation reactions of mono-, di-, and triesters of ...
2012-12-07 [J. Org. Chem. 77(23) , 10907-13, (2012)] |
|
Nanomolar inhibition of the enterobactin biosynthesis enzyme...
2006-07-15 [Bioorg. Med. Chem. Lett. 16(14) , 3802-5, (2006)] |
|
Inhibition of chymotrypsin by a complex of ortho-vanadate an...
2007-05-22 [Biochemistry 46(20) , 5982-90, (2007)] |