Double diastereoselection in aldol reactions mediated by dicyclohexylchloroborane between chiral aldehydes and a chiral ethyl ketone derived from L-erythrulose. synthesis of a C1-C9 fragment of the structure of the antifungal metabolite soraphen A1alpha.
Santiago Díaz-Oltra, Juan Murga, Eva Falomir, Miguel Carda, Gabriel Peris, J Alberto Marco
文献索引:J. Org. Chem. 70 , 8130-8139, (2005)
全文:HTML全文
摘要
[Chemical reaction: See text] Both matched and mismatched diastereoselections have been observed in the aldol reactions of a range of chiral aldehydes with the dicyclohexylboron enolate of a chiral ethyl ketone related to L-erythrulose. As was previously observed in the corresponding aldol reactions with L-erythrulose derivatives, the Felkin-Anh model provides an adequate explanation for the stereochemical outcome of reactions with chiral alpha-methyl aldehydes. However, a satisfactory account of the results observed with alpha-oxygenated aldehydes was only possible with the Cornforth model. As a practical application of the methodology described herein, a C1-C9 fragment of the structure of the antifungal macrolide soraphen A1alpha has been prepared in a convergent and stereoselective way.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
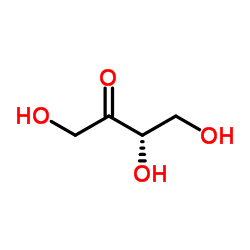 |
L-赤藓酮糖
CAS:533-50-6 |
C4H8O4 |
|
Double diastereoselection in aldol reactions mediated by dic...
2003-10-31 [J. Org. Chem. 68 , 8577-8582, (2003)] |
|
Aldol reactions between L-erythrulose derivatives and chiral...
2008-01-01 [Chemistry 14 , 9240-9254, (2008)] |
|
Characterization and inactivation of the membrane-bound poly...
2010-06-01 [Microbiology 156 , 1890-1899, (2010)] |
|
Identification of novel thermostable taurine-pyruvate transa...
2016-04-01 [Appl. Microbiol. Biotechnol. 100 , 3101-11, (2016)] |
|
The non-oxidative degradation of ascorbic acid at physiologi...
2000-04-15 [Biochim. Biophys. Acta 1501(1) , 12-24, (2000)] |