| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
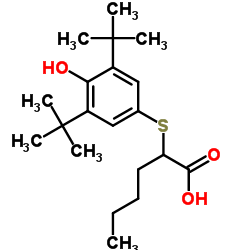 |
肌酸酐脱亚氨酶
CAS:37289-15-9 |
|
 |
SARCOSINE DEHYDROGENASE
CAS:37228-65-2 |