Luteinization-associated changes in protein stability of the regulatory subunit of the type I cAMP-dependent protein kinase.
V Jackiw, M Hunzicker-Dunn
文献索引:J. Biol. Chem. 267(20) , 14335-44, (1992)
全文:HTML全文
摘要
The purpose of this study was to determine how RI alpha, the R subunit of the type I cAMP-dependent protein kinase, is regulated in rabbit ovarian follicles in response to the preovulatory luteinizing hormone surge. When soluble extracts from rabbit preovulatory follicles and 7-day-old corpora lutea were photoaffinity-labeled with 8-N3-[32P]cAMP, 3-fold more RI alpha was detected in corpora lutea than in follicles. Based on DEAE-cellulose chromatography, both type I holoenzyme and free RI alpha increased during luteinization. Western blot analysis of soluble extracts obtained from follicles and corpora lutea at various time points after human chorionic gonadotropin (hCG) injection revealed a 6-10-fold increase in RI alpha protein by 5 h after hCG injection. However, based on Northern blot analysis and solution hybridization/RNase protection assays, this increase in RI alpha protein was not due to an increase in RI alpha mRNA. These results suggested that RI alpha subunit levels were post-transcriptionally regulated. Half-life determinations indicated a 2.1-fold increase in the stability of RI alpha when follicles were incubated in the presence of hCG. The effect of hCG on the stability of RI alpha could also be mimicked by forskolin, thus suggesting that a rise in cAMP levels in follicles during the luteinizing hormone surge plays a role in RI alpha subunit stability. We conclude that RI alpha protein is stabilized in follicles by hCG treatment and the consequent rise in follicular cAMP levels.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
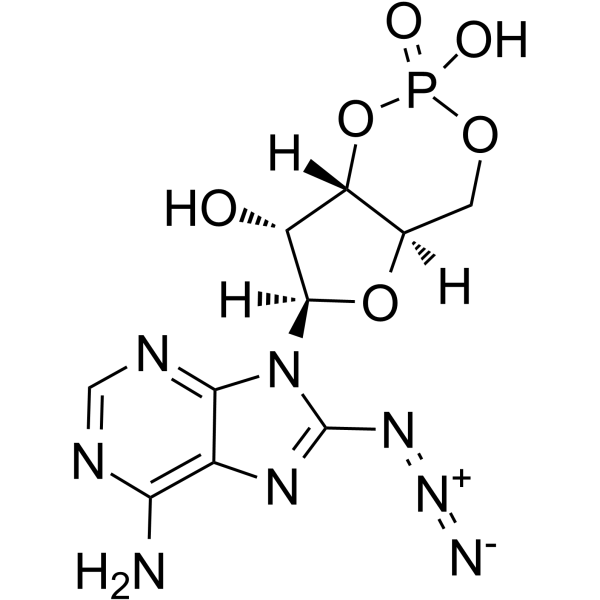 |
8-叠氮腺苷3':5'-环一磷酸
CAS:31966-52-6 |
C10H11N8O6P |
|
The covalent labeling of proteins by 17 beta-estradiol, reti...
1992-11-01 [J. Steroid Biochem. Mol. Biol. 43(6) , 489-97, (1992)] |
|
A highly decreased binding of cyclic adenosine monophosphate...
2002-07-01 [J. Invest. Dermatol. 119(1) , 160-5, (2002)] |
|
Lipolytic membrane release of two phosphatidylinositol-ancho...
1994-02-01 [Arch. Biochem. Biophys. 308(2) , 504-14, (1994)] |
|
Post-translational modifications of the regulatory subunits ...
2001-01-01 [C. R. Acad. Sci. III 324(1) , 23-31, (2001)] |
|
Cyclic AMP-dependent protein kinase in rat mammary tissue: e...
1994-08-01 [Biochem. J. 301 ( Pt 3) , 807-12, (1994)] |