Mechanistic investigations of human reticulocyte 15- and platelet 12-lipoxygenases with arachidonic acid.
Aaron T Wecksler, Cyril Jacquot, Wilfred A van der Donk, Theodore R Holman
文献索引:Biochemistry 48(26) , 6259-67, (2009)
全文:HTML全文
摘要
Human reticulocyte 15-lipoxygenase-1 (15-hLO-1) and human platelet 12-lipoxygenase (12-hLO) have been implicated in a number of diseases, with differences in their relative activity potentially playing a central role. In this work, we characterize the catalytic mechanism of these two enzymes with arachidonic acid (AA) as the substrate. Using variable-temperature kinetic isotope effects (KIE) and solvent isotope effects (SIE), we demonstrate that both k(cat)/K(M) and k(cat) for 15-hLO-1 and 12-hLO involve multiple rate-limiting steps that include a solvent-dependent step and hydrogen atom abstraction. A relatively low k(cat)/K(M) KIE of 8 was determined for 15-hLO-1, which increases to 18 upon the addition of the allosteric effector molecule, 12-hydroxyeicosatetraenoic acid (12-HETE), indicating a tunneling mechanism. Furthermore, the addition of 12-HETE lowers the observed k(cat)/K(M) SIE from 2.2 to 1.4, indicating that the rate-limiting contribution from a solvent sensitive step in the reaction mechanism of 15-hLO-1 has decreased, with a concomitant increase in the C-H bond abstraction contribution. Finally, the allosteric binding of 12-HETE to 15-hLO-1 decreases the K(M)[O(2)] for AA to 15 microM but increases the K(M)[O(2)] for linoleic acid (LA) to 22 microM, such that the k(cat)/K(M)[O(2)] values become similar for both substrates (approximately 0.3 s(-1) microM(-1)). Considering that the oxygen concentration in cancerous tissue can be less than 5 microM, this result may have cellular implications with respect to the substrate specificity of 15-hLO-1.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
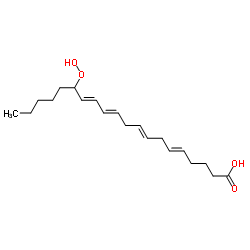 |
15-羟基二十碳-5Z,8Z,11Z,13E-四烯酸
CAS:70981-96-3 |
C20H32O4 |
|
The human serum metabolome.
2011-01-01 [PLoS ONE 6(2) , e16957, (2011)] |
|
Novel role of lipoxygenases in the inflammatory response: pr...
2005-03-15 [J. Immunol. 174(6) , 3169-72, (2005)] |
|
Nature of the cardiomyocyte injury induced by lipid hydroper...
1995-11-01 [Cardiovasc. Res. 30(5) , 648-55, (1995)] |
|
On the relationships of substrate orientation, hydrogen abst...
2005-11-18 [J. Biol. Chem. 280(46) , 38756-66, (2005)] |
|
Liquid chromatography/mass spectrometry analysis of bifuncti...
2005-01-01 [Rapid Commun. Mass Spectrom. 19(6) , 849-58, (2005)] |