THz spectra and dynamics of aqueous solutions studied by the ultrafast optical Kerr effect.
Kamila Mazur, Ismael A Heisler, Stephen R Meech
文献索引:J. Phys. Chem. B 115(11) , 2563-73, (2011)
全文:HTML全文
摘要
The nature and extent of the effects that hydrophilic and hydrophobic solutes have on the dynamics of water molecules continues to be an area of intense experimental and theoretical investigation. In this work, we use the ultrafast optical Kerr effect to measure the picosecond dynamics and THz Raman spectral densities of a series of aqueous solutions. The solutes studied are the hydrophilic urea and formamide and the hydrophobic trimethylamine N-oxide and tetramethylurea. Measurements are made as a function of concentration between <0.1 M and >4 M. At low concentrations (<0.5 M), the THz spectrum resembles that of bulk water, but the picosecond relaxation time, reflecting dynamics in the water H-bonded network, is increased relative to bulk water for all four solutes. The extent to which water relaxation is slowed down depends on the nature of the solute, and is more pronounced for hydrophilic than for hydrophobic solutes. At concentrations above 1 M, a range of solute-solvent and solute-solute interactions gives rise to diverse solute dependent changes in the THz spectral density and to a further slowing down of the picosecond relaxation. The hydrophobic trimethylamine N-oxide has remarkably little effect on the spectral density of water, which may indicate solute self-association and the formation of water pools in more concentrated solutions. For hydrophilic urea and formamide, the THz spectral density suggests that water structure is disrupted at concentrations where most water molecules are part of a solvation shell. At such high concentrations, modes associated with the H-bonded solute make a significant contribution to the spectral density at around 100 cm(-1). The hydrophobic tetramethylurea solute makes a substantial contribution to the spectral density, complicating the interpretation, but a line shape analysis suggests that it also does not strongly perturb the water structure.© 2011 American Chemical Society
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
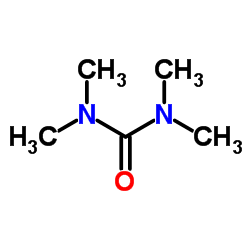 |
四甲基脲
CAS:632-22-4 |
C5H12N2O |
|
Transformation of NIH3T3 mouse fibroblast cells by MUC16 muc...
2015-01-01 [Int. J. Oncol. 46(1) , 91-8, (2014)] |
|
Coordination chemistry study of hydrated and solvated lead(I...
2011-02-07 [Inorg. Chem. 50(3) , 1058-72, (2011)] |
|
Solvent-induced lysozyme gels: rheology, fractal analysis, a...
2005-09-15 [J. Colloid. Interface Sci. 289(2) , 394-401, (2005)] |
|
Strong slowing down of water reorientation in mixtures of wa...
2008-03-20 [J. Phys. Chem. A 112(11) , 2355-61, (2008)] |
|
The hydrogen bond network structure within the hydration she...
2010-07-21 [J. Chem. Phys. 133(3) , 035102, (2010)] |