Evidence for the existence of both proteasomes and a novel high molecular weight peptidase in Entamoeba histolytica.
H Scholze, S Frey, Z Cejka, T Bakker-Grunwald
文献索引:J. Biol. Chem. 271(11) , 6212-6, (1996)
全文:HTML全文
摘要
To screen for high molecular weight proteases in Entamoeba histolytica, we subjected a soluble amebal extract to density gradient centrifugation and tested the fractions for activity against the chymotryptic peptide substrate, Suc-leucyl-leucyl-valyl-tyrosyl-4-methylcoumaryl-7-amide. Two peaks of activity, of approximately 11 and 20 S, were clearly separated. Based on SDS-electrophoretic pattern and immunoblot analysis, we ascribe the 20 S activity to proteasomes. The 11 S protein was purified from amebal homogenates by a series of chromatographic steps. As determined by molecular sieve chromatography and nondenaturing gel electrophoresis, the native complex had an apparent Mr of 385,000 +/- 10%. On SDS gels, the purified enzyme exhibited a single band of Mr 62,000 that yielded a single N-terminal sequence, indicating that the preparation was homogeneous and that the native complex consisted of six very similar or identical subunits. The enzyme preferred peptides with aromatic residues at the P1 position and had low but distinct activity toward azocasein. We conclude that the 11 S enzyme is a novel high molecular weight protease that is distinct from proteasomes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
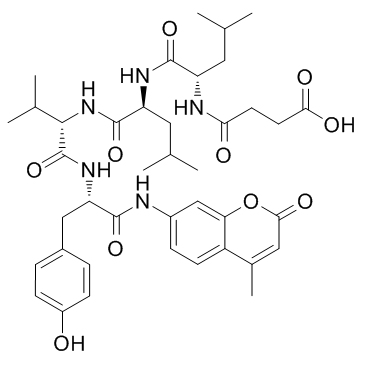 |
SUC-LEU-LEU-VAL-酪氨酸-AMC
CAS:94367-21-2 |
C40H53N5O10 |
|
Human high temperature requirement serine protease A1 (HTRA1...
2012-06-15 [J. Biol. Chem. 287(25) , 20931-41, (2012)] |
|
Turnover of oxidatively damaged nuclear proteins in BV-2 mic...
2001-06-01 [FASEB J. 15(8) , 1460-2, (2001)] |
|
Activation of the cell death program by nitric oxide involve...
1999-07-09 [J. Biol. Chem. 274(28) , 19581-6, (1999)] |
|
Differential impairment of 20S and 26S proteasome activities...
2000-05-01 [Arch. Biochem. Biophys. 377(1) , 65-8, (2000)] |
|
The Leishmania mexicana proteasome.
1999-09-20 [Mol. Biochem. Parasitol. 103(1) , 49-60, (1999)] |