CYP2E1 degradation by in vitro reconstituted systems: role of the molecular chaperone hsp90.
T Goasduff, A I Cederbaum
文献索引:Arch. Biochem. Biophys. 379(2) , 321-30, (2000)
全文:HTML全文
摘要
One major mode of regulation of cytochrome P450 2E1 (CYP2E1) is at the posttranscriptional level, since many low-molecular-weight compounds stabilize the enzyme against proteolysis by the proteasome complex. In an in vitro system containing human liver microsomes, degradation of CYP2E1 in the microsomes required addition of the human liver cytosol fraction in a reaction sensitive to inhibitors of the proteasome complex. It is not clear how CYP2E1 in the microsomal membrane becomes accessible to the cytosolic proteasome. Since molecular chaperones play a role in protein folding and degradation, the possible role of heat shock proteins in CYP2E1 degradation by this reconstituted system was evaluated. Degradation of CYP2E1 required ATP; ATP-gammaS, a nonhydrolyzable analogue of ATP, did not catalyze CYP2E1 degradation by the cytosol fraction, indicating that ATP hydrolysis is required. Geldanamycin, a specific inhibitor of hsp90, inhibited the degradation of microsomal CYP2E1 by the cytosol fraction. Control experiments indicated that geldanamycin was not a substrate/ligand of CYP2E1 nor did it prevent microsomal lipid peroxidation, a process which increases CYP2E1 turnover. Inhibition by geldanamycin was prevented by molybdate. Both of these compounds have been shown to promote alterations in hsp90 structure and to modulate hsp90-protein interactions. The proteasome activity in the cytosol, as assayed by the cleavage of a fluorogenic peptide, was enhanced when ATP was added and inhibited by 30-40% by geldanamycin, effects that are similar, although less pronounced, to the degradation of CYP2E1 by the cytosol. Purified 20S proteasome could catalyze degradation of CYP2E1; however, in an assay using equal peptidase activity, the cytosol fraction was much more effective than the 20S proteasome in promoting CYP2E1 degradation. Immunodepletion of hsp90 from the cytosol resulted in prevention of the degradation of CYP2E1, a reaction that was reversed by the addition of pure hsp90 to this cytosol. These results suggest that in addition to the proteasome, the cytosol fraction contains other factors that modulate the efficiency of CYP2E1 degradation. The sensitivity to geldanamycin and molybdate and the immunodepletion experiments suggest that hsp90 is one of these factors that interact with CYP2E1 and/or with the proteasome to promote the degradation of this microsomal P450.Copyright 2000 Academic Press.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
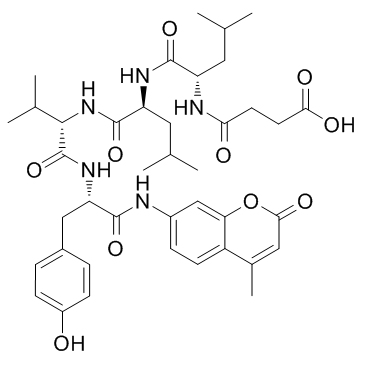 |
SUC-LEU-LEU-VAL-酪氨酸-AMC
CAS:94367-21-2 |
C40H53N5O10 |
|
Human high temperature requirement serine protease A1 (HTRA1...
2012-06-15 [J. Biol. Chem. 287(25) , 20931-41, (2012)] |
|
Turnover of oxidatively damaged nuclear proteins in BV-2 mic...
2001-06-01 [FASEB J. 15(8) , 1460-2, (2001)] |
|
Activation of the cell death program by nitric oxide involve...
1999-07-09 [J. Biol. Chem. 274(28) , 19581-6, (1999)] |
|
Differential impairment of 20S and 26S proteasome activities...
2000-05-01 [Arch. Biochem. Biophys. 377(1) , 65-8, (2000)] |
|
The Leishmania mexicana proteasome.
1999-09-20 [Mol. Biochem. Parasitol. 103(1) , 49-60, (1999)] |