| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
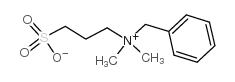 |
3-(苄基二甲基铵基)丙烷基磺酸
CAS:81239-45-4 |
|
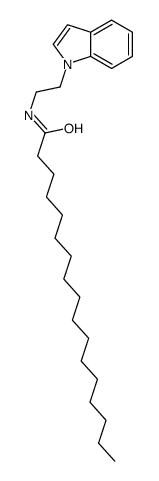 |
十七烷酸色酰胺
CAS:232257-97-5 |