Inhibition of fatty acid oxidation by 2-bromooctanoate. Evidence for the enzymatic formation of 2-bromo-3-ketooctanoyl coenzyme A and the inhibition of 3-ketothiolase.
B M Raaka, J M Lowenstein
文献索引:J. Biol. Chem. 254(14) , 6755-62, (1979)
全文:HTML全文
摘要
Incubation of rat liver mitochondria with 10 microM DL-2-bromooctanoate causes complete and irreversible inactivation of 3-ketothiolase I (acyl-CoA:acetyl-CoA C-acyltransferase). Evidence is presented that mitochondria convert bromooctanoate to 2-bromo-3-ketooctanoyl-CoA, an alpha-haloketone which is probably the active form of the inhibitor. The inactivation is accompanied by incorporation of radioactivity from [1-14C]bromooctanoate into the enzyme. Bromooctanoate does not affect the activities of the other enzymes of beta-oxidation, except for 3-ketothiolase II (acetyl-CoA:acetyl-CoA C-acetyltransferase), which becomes partially inhibited. Evidence is also presented that various enzymes of beta-oxidation can use 2-bromooctanoyl-CoA and its beta-oxidation products as substrates.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
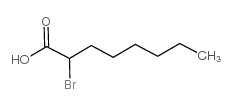 |
2-溴正辛酸
CAS:2623-82-7 |
C8H15BrO2 |
|
Accumulation of polyhydroxyalkanoic acid containing large am...
2001-11-01 [Appl. Environ. Microbiol. 67(11) , 4963-74, (2001)] |
|
Hyperpolarization of the cell membrane of mouse hepatocytes ...
1995-03-01 [Physiol. Behav. 57(3) , 509-14, (1995)] |
|
Hexanoate and octanoate inhibit transcription of the malic e...
1992-07-25 [J. Biol. Chem. 267(21) , 14918-27, (1992)] |
|
The involvement of carnitine intermediates in peroxisomal fa...
1990-09-01 [Arch. Biochem. Biophys. 281(2) , 233-8, (1990)] |
|
Effects of inhibitors of diacylglycerol metabolism on protei...
1988-06-08 [Biochim. Biophys. Acta 970(1) , 68-74, (1988)] |