Effect of urea on the ion-association capillary electrophoresis separation of the anionic metal complexes using hydrophobic tetraalkylammonium as ion-association agent.
Toru Takahashi, Hitoshi Hoshino
文献索引:Anal. Sci. 26(11) , 1151-6, (2010)
全文:HTML全文
摘要
The effect of urea as an electrophoretic buffer solution modifier on the ion-association (IA) capillary electrophoresis (CE) separation of four anionic metal complexes of Al(III), Co(III), Cr(III), and Fe(III) with 2,2'-dihydroxyazobenezene-5,5'-disulfonate (DHABS) using a hydrophobic ion-association agent, tetrapentylammonium bromide, was studied. The mutual separation of the four anionic metal-DHABS complexes was not achieved without the addition of urea in the electrophoretic buffer solution. However, the addition of 1.5 M urea in the electrophoretic buffer solution brought about a complete separation of the four metal complexes. The ion-association constants between all metal-DHABS complexes and tetrapentylammonium in an aqueous urea solution were smaller than those in a neat aqueous solution. This indicates the hydrophobic interaction contributing to the ion-association between analytes and ion-association agent during IA-CE separation processes can be controlled with the addition of urea to the electrophoretic buffer solution. Another advantage of adding urea was a substantial enhancement of separation efficiency with a reduction of the half-bandwidth of the peaks. Also, a reduction of the electrophoretic mobility of the electroosmotic flow when urea was added was much less than when organic solvents were used.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
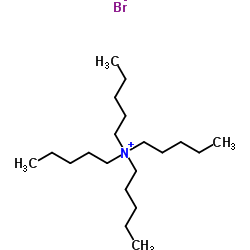 |
四戊基溴化铵
CAS:866-97-7 |
C20H44BrN |
|
KCNE1 reverses the response of the human K+ channel KCNQ1 to...
2000-12-01 [Pflugers Arch. 441(2-3) , 368-78, (2000)] |
|
Early-phase occurrence of K+ and Cl- efflux in addition to C...
2012-08-01 [Apoptosis 17(8) , 821-31, (2012)] |
|
Frequency dependence of alternating current electrospray ion...
2011-04-15 [Anal. Chem. 83(8) , 3017-23, (2011)] |
|
Endothelium-dependent relaxation by tetraoctylammonium ions ...
2000-01-01 [Life Sci. 66(1) , PL13-9, (2000)] |
|
Tetrapentylammonium block of chloramine-T and veratridine mo...
2001-04-01 [Br. J. Pharmacol. 132(8) , 1755-60, (2001)] |