Mass spectrometric behavior of thiazide-based diuretics after electrospray ionization and collision-induced dissociation.
Mario Thevis, M Hans Schmickler, Wilhelm Schänzert
文献索引:Anal. Chem. 74(15) , 3802-8, (2002)
全文:HTML全文
摘要
The mass spectrometric behavior of 21 thiazide-based compounds after electrospray ionization in the negative ion mode and collision-induced dissociation was investigated on a triple-stage quadrupole mass spectrometer. The mass spectra show individual and common fragmentation patterns, the generations of which are discussed based on comparable molecular structures of commercially available substances and the synthesis of unlabeled, deuterated, and 15N-labeled analogues. The synthesis of deuterated thiazides is perfomed by condensation of 4-amino-6-chloro-1,3-benzenedisulfonamide with appropriately labeled aldehydes, while the introduction of 15N into the sulfonamide groups of thiazides was achieved by the synthesis of 4-amino-6-chloro-1,3-benzenedisulfonamide(15N2) from 3-chloroaniline via 4-amino-6-chloro-1,3-benzenedisulfonyl chloride. The most common fragments determined are m/z 269, 205, and 126 for 6-chloro-7-sulfamoyl-3-alkyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxides and m/z 303, 239, and 160 for 6-trifluoromethyl-7-sulfamoyl-3-alkyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxides. Individual fragmentation behaviors were found that mainly depended on the C-3-linked side chain.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
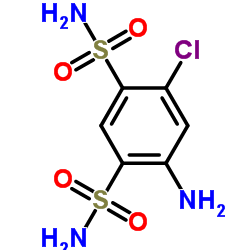 |
4-氨基-6-氯-1,3-苯二磺酰胺
CAS:121-30-2 |
C6H8ClN3O4S2 |
|
Carbonic anhydrase inhibitors: cloning and sulfonamide inhib...
2006-04-15 [Bioorg. Med. Chem. Lett. 16 , 2182-8, (2006)] |
|
Detection of urinary markers for thiazide diuretics after or...
2009-01-01 [J. Chromatogr. A. 1216(12) , 2466-73, (2009)] |
|
Validated micellar electrokinetic capillary chromatography m...
1994-07-15 [J. Chromatogr. B, Biomed. Appl. 657(2) , 383-94, (1994)] |
|
Biopharmaceutical studies of thiazide diuretics. III. In viv...
1987-08-01 [Chem. Pharm. Bull. 35(8) , 3516-8, (1987)] |
|
Binding of 2-amino-4-chloro-m-benzenedisulfonamide as a meta...
1990-10-01 [Chem. Pharm. Bull. 38(10) , 2882-3, (1990)] |