Journal of the American Chemical Society
2008-12-17
Metal-mediated cyclization of aryl and benzyl glycidyl ethers: a complete scenario.
Rocío Marcos, Carles Rodríguez-Escrich, Clara I Herrerías, Miquel A Pericàs
文献索引:J. Am. Chem. Soc. 130(50) , 16838-9, (2008)
全文:HTML全文
摘要
Enantiomerically pure aryl and benzyl glycidyl ethers undergo stereospecific cyclizations leading to 3-chromanols or to tetrahydrobenzo[c]oxepin-4-ols under mild conditions in the presence of a catalytic amount of FeBr3/3AgOTf. The same processes are also mediated, albeit in a much less efficient manner, by AuCl3/3AgOTf. The set of active mediators in this group of processes (BF3 x Et2O, FeBr3, FeBr3/3AgOTf, AuCl3/3AgOTf) shows its nature as Friedel-Crafts reactions and disfavors the intermediacy of arylgold species.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
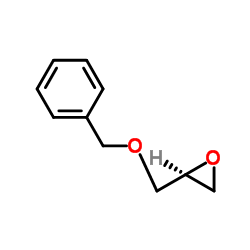 |
(S)-苄氧甲基环氧乙烷
CAS:16495-13-9 |
C10H12O2 |
相关文献:
更多...
|
Bioresolution of benzyl glycidyl ether using whole cells of ...
2012-08-01 [J. Basic Microbiol. 52(4) , 383-9, (2012)] |
|
Enantioselective total synthesis of (+)-gigantecin: exploiti...
2004-10-13 [J. Am. Chem. Soc. 126(40) , 12790-1, (2004)] |
|
S. Takano et al.
[Tetrahedron Lett. 31 , 3619, (1990)] |
|
R.E. Ireland et al.
[J. Org. Chem. 55 , 1423, (1990)] |
|
M. Honda et al.
[Chem. Pharm. Bull. 39 , 1385, (1991)] |