A molecular docking strategy identifies Eosin B as a non-active site inhibitor of protozoal bifunctional thymidylate synthase-dihydrofolate reductase.
Chloé E Atreya, Eric F Johnson, John J Irwin, Antonia Dow, Kristen M Massimine, Isabelle Coppens, Valeska Stempliuk, Stephen Beverley, Keith A Joiner, Brian K Shoichet, Karen S Anderson
文献索引:J. Biol. Chem. 278(16) , 14092-100, (2003)
全文:HTML全文
摘要
Protozoal parasites are unusual in that their thymidylate synthase (TS) and dihydrofolate reductase (DHFR) enzymes exist on a single polypeptide. In an effort to probe the possibility of substrate channeling between the TS and DHFR active sites and to identify inhibitors specific for bifunctional TS-DHFR, we used molecular docking to screen for inhibitors targeting the shallow groove connecting the two active sites. Eosin B is a 100 microm non-active site inhibitor of Leishmania major TS-DHFR identified by molecular docking. Eosin B slows both the TS and DHFR reaction rates. When Arg-283, a key residue to which eosin B is predicted to bind, is mutated to glutamate, however, eosin B only minimally inhibits the TS-DHFR reaction. Additionally, eosin B was found to be a 180 microm inhibitor of Toxoplasma gondii in both biochemical and cell culture assays.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
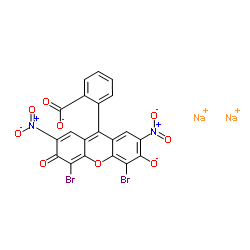 |
曙红B
CAS:548-24-3 |
C20H6Br2N2Na2O9 |
|
Modulation of collagen by addition of Hofmeister salts.
2015-08-01 [Int. J. Biol. Macromol. 79 , 518-26, (2015)] |
|
How Romanowsky stains work and why they remain valuable - in...
2011-02-01 [Biotech. Histochem. 86(1) , 36-51, (2011)] |
|
Functional characterization of the domains of the bovine bin...
2015-01-01 [Reprod. Biol. Endocrinol. 13 , 64, (2015)] |
|
In vivo pharmacology and antidiarrheal efficacy of a thiazol...
2005-01-01 [J. Microbiol. Methods 116 , 1-7, (2015)] |
|
Prevention of phenytoin-induced gingival overgrowth by lovas...
2015-06-01 [Am. J. Pathol. 185 , 1588-99, (2015)] |