Luminescence studies of decomposition of ceric sulfate.
M A Bakane, C P Joshi, S V Moharil, P L Muthal, S M Dhopte
文献索引:Luminescence 26(6) , 553-6, (2011)
全文:HTML全文
摘要
Literature results on the decomposition products of ceric sulfate are inconsistent. A group of researchers claim that ceric sulfate decomposed to ceric oxide without going through a cerous phase at any stage, while the results of the other group show that cerous sulfate is formed as an intermediate phase. Most of these studies used DTA/TGA, XRD and IR techniques. Cerous compounds can also be detected by the characteristic luminescence of Ce(3+). Using such techniques we show that the thermal decomposition of both monoclinic and βCe(SO(4) )(2) · 4H(2) O in air at 500°C leads to the formation of cerous sulphate. Use of various atmospheres (air/N(2) /vacuum) and temperature profiles for the decomposition by the different researchers may be responsible for the discrepancies between literature results.Copyright © 2011 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
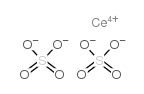 |
硫酸铈(IV)
CAS:13590-82-4 |
CeO8S2 |
|
Differential in vitro inhibition studies of some cerium vana...
2015-04-01 [J. Enzyme Inhib. Med. Chem. 30(2) , 286-9, (2015)] |
|
Microsphaerol and seimatorone: two new compounds isolated fr...
2015-02-01 [Chem. Biodivers. 12(2) , 289-94, (2015)] |
|
Oxidation, deformation, and destruction of carbon nanotubes ...
2005-02-03 [J. Phys. Chem. B 109(4) , 1400-7, (2005)] |
|
Investigation of the NMR relaxation rate dose-response of a ...
2002-06-01 [Appl. Radiat. Isot. 56(6) , 895-9, (2002)] |
|
Chemiluminescence determination of ferulic acid by flow-inje...
2008-11-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 71(1) , 204-8, (2008)] |