Short, multiple-stranded β-hairpin peptides have antimicrobial potency with high selectivity and salt resistance.
Shuli Chou, Changxuan Shao, Jiajun Wang, Anshan Shan, Lin Xu, Na Dong, Zhongyu Li
文献索引:Acta Biomater. 30 , 78-93, (2015)
全文:HTML全文
摘要
The β-hairpin structure has been proposed to exhibit potent antimicrobial properties with low cytotoxicity, thus, multiple β-hairpin structures have been proved to be highly stable in structures containing tightly packed hydrophobic cores. The aim of this study was to develop peptide-based synthetic strategies for generating short, but effective AMPs as inexpensive antimicrobial agents. Multiple-stranded β-hairpin peptides with the same β-hairpin unit, (WRXxRW)n where n=1, 2, 3, or 4 and Xx represent the turn sequence, were synthesized, and their potential as antimicrobial agents was evaluated. Owning to the tightly packed hydrophobic core and paired Trp of this multiple-stranded β-hairpin structure, all the 12-residues peptides exhibited high cell selectivity towards bacterial cells over human red blood cells (hRBCs), and the peptide W2 exhibited stronger antimicrobial activities with the MIC values of 2-8μM against various tested bacteria. Not only that, but W2 also showed obvious synergy with streptomycin and chloramphenicol against Escherichia coli, and displayed synergy with ciprofloxacin against Staphylococcus aureus with the FICI values ⩽0.5. Fluorescence spectroscopy and electron microscopy analyses indicated that W2 kills microbial cells by permeabilizing the cell membrane and damaging membrane integrity. Collectively, based on the multiple β-hairpin peptides, the ability to develop libraries of short and effective peptides will be a powerful approach to the discovery of novel antimicrobial agents.We successfully screened a peptide W2 ((WRPGRW)2) from a series of multiple-stranded β-hairpin antimicrobial peptides based on the "S-shaped" motif that induced the formation of a globular structure, and Trp zipper was used to replace the disulfide bonds to reduce the cost of production. This novel structure applied to AMPs improved cell selectivity and salt stability. The findings of this study will promote the development of peptide-based antimicrobial biomaterials. Further exploration of these AMPs will allow for diverse biotechnological and clinical applications such as biomedical coating, food storaging, and animal feeding.Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
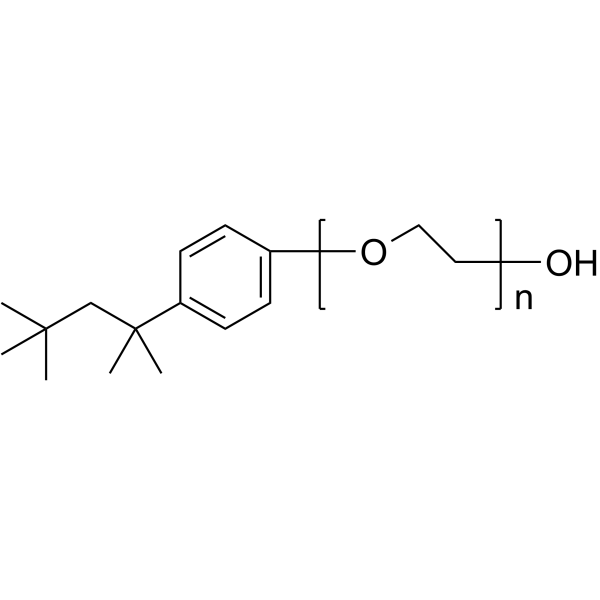 |
曲拉通X-100
CAS:9002-93-1 |
(C2H4O)n.C14H22O | |
 |
3,3'-二丙基硫杂二羰花青碘化物
CAS:53213-94-8 |
C25H27IN2S2 |
|
A survey of the interactome of Kaposi's sarcoma-associated h...
2015-05-01 [J. Virol. 89(9) , 4918-31, (2015)] |
|
Phospho-tyrosine dependent protein-protein interaction netwo...
2015-03-01 [Mol. Syst. Biol. 11(3) , 794, (2015)] |
|
The combination of electric current and copper promotes neur...
2015-04-01 [Ann. Biomed. Eng. 43(4) , 1014-23, (2015)] |
|
A precisely substituted benzopyran targets androgen refracto...
2015-03-15 [Toxicol. Appl. Pharmacol. 283(3) , 187-97, (2015)] |
|
Involvement of epigenetics and EMT-related miRNA in arsenic-...
2015-03-01 [Cancer Prev. Res. (Phila.) 8(3) , 208-21, (2015)] |