Synthesis and pharmacological evaluation of N-acyl-1,2,3,4-tetrahydroisoquinoline derivatives as novel specific bradycardic agents.
Hideki Kubota, Toshihiro Watanabe, Akio Kakefuda, Noriyuki Masuda, Kouichi Wada, Noe Ishii, Shuichi Sakamoto, Shinichi Tsukamoto
文献索引:Bioorg. Med. Chem. 12(5) , 871-82, (2004)
全文:HTML全文
摘要
A series of N-acyl-1,2,3,4-tetrahydroisoquinoline derivatives were synthesized and evaluated for their bradycardic activities in isolated guinea pig right atria and in urethane-anesthetized rats. These efforts resulted in identification of the compound 8a, which exhibits potent bradycardic activity with minimal influence on mean blood pressure in urethane-anesthetized rats. Oral administration of compound 8a to conscious rats revealed increased potency and prolonged duration of action when compared to Zatebradine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
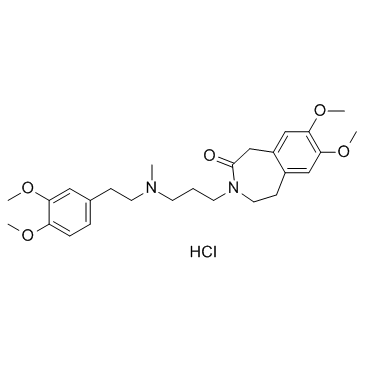 |
盐酸Zatebradine
CAS:91940-87-3 |
C26H37ClN2O5 |
|
Abnormal diastolic currents in ventricular myocytes from spo...
2006-11-01 [Am. J. Physiol. Heart Circ. Physiol. 291(5) , H2192-8, (2006)] |
|
Effects of tachycardia on regional wall motion in acute isch...
2004-06-01 [Tohoku J. Exp. Med. 203(2) , 111-21, (2004)] |
|
Validation of a high-sensitivity assay for zatebradine in dr...
2010-11-01 [Bioanalysis 2(11) , 1863-71, (2010)] |
|
Heart rate reduction by zatebradine reduces infarct size and...
2004-04-01 [Am. J. Physiol. Heart Circ. Physiol. 286(4) , H1281-8, (2004)] |
|
Blocking effects of acehytisine on pacemaker currents (I(f))...
2012-01-06 [J. Ethnopharmacol. 139(1) , 42-51, (2012)] |