| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
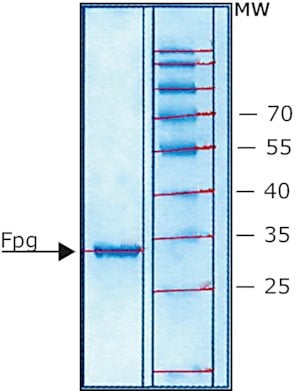 |
Fpg Protein from Escherichia coli
CAS:78783-53-6 |
|
 |
Polynucleotide kinase
CAS:37211-65-7 |