Chemically engineered papain as artificial formate dehydrogenase for NAD(P)H regeneration.
Pierre Haquette, Barisa Talbi, Laure Barilleau, Nathalie Madern, Céline Fosse, Michèle Salmain
文献索引:Org. Biomol. Chem. 9(16) , 5720-7, (2011)
全文:HTML全文
摘要
Organometallic complexes of the general formula [(η(6)-arene)Ru(N⁁N)Cl](+) and [(η(5)-Cp*)Rh(N⁁N)Cl](+) where N⁁N is a 2,2'-dipyridylamine (DPA) derivative carrying a thiol-targeted maleimide group, 2,2'-bispyridyl (bpy), 1,10-phenanthroline (phen) or ethylenediamine (en) and arene is benzene, 2-chloro-N-[2-(phenyl)ethyl]acetamide or p-cymene were identified as catalysts for the stereoselective reduction of the enzyme cofactors NAD(P)(+) into NAD(P)H with formate as a hydride donor. A thorough comparison of their effectiveness towards NAD(+) (expressed as TOF) revealed that the Rh(III) complexes were much more potent catalysts than the Ru(II) complexes. Within the Ru(II) complex series, both the N⁁N and arene ligands forming the coordination sphere had a noticeable influence on the activity of the complexes. Covalent anchoring of the maleimide-functionalized Ru(II) and Rh(III) complexes to the cysteine endoproteinase papain yielded hybrid metalloproteins, some of them displaying formate dehydrogenase activity with potentially interesting kinetic parameters.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
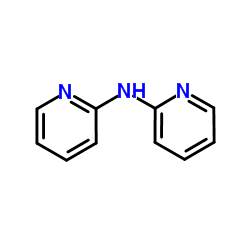 |
2,2'-二吡啶胺
CAS:1202-34-2 |
C10H9N3 |
|
Synthesis, characterization, DNA interaction and cleavage, a...
2011-10-07 [Dalton Trans. 40(37) , 9404-12, (2011)] |
|
Third generation fluoroquinolones antibacterial drug based m...
2011-04-01 [J. Enzyme Inhib. Med. Chem. 26(2) , 188-97, (2011)] |
|
Concurrent induction of rat hepatic microsomal cytochrome P4...
2000-07-01 [Xenobiotica 30(7) , 683-92, (2000)] |
|
Synthesis, radiolabeling, and bio-imaging of high-generation...
2009-03-04 [J. Am. Chem. Soc. 131(8) , 2906-16, (2009)] |
|
Syntheses, structures, and luminescent properties of dipyrid...
2013-02-01 [Inorg. Chem. 51(13) , 7039-49, (2012)] |