Side-chain peptide-synthetic polymer conjugates via tandem "ester-amide/thiol-ene" post-polymerization modification of poly(pentafluorophenyl methacrylate) obtained using ATRP.
Nikhil K Singha, Matthew I Gibson, Bishnu P Koiry, Maarten Danial, Harm-Anton Klok
文献索引:Biomacromolecules 12(8) , 2908-13, (2011)
全文:HTML全文
摘要
Herein the concept of tandem postpolymerization modification as a versatile route to synthesize well-defined, highly functionalized polymers is introduced. Poly(pentafluorophenyl methacrylate) obtained by atom transfer radical polymerization was first modified with allylamine, which displaces the active ester to give well-defined polymers with pendant alkene groups, which are difficult to obtain by direct (radical) polymerization of allylic-functional monomers. The produced poly(allylmethacrylamide) was modified by a second postpolymerization modification reaction with a thiol-terminated peptide (CVPGVG) using AIBN as the radical source. NMR, IR, and SEC demonstrated successful conjugation onto the polymer to give a polymer-peptide hybrid material. This versatile strategy should extend the scope of controlled radical polymerization and "click"-type reactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
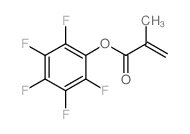 |
甲基丙烯酸五氟苯酯
CAS:13642-97-2 |
C10H5F5O2 |
|
Detection of Peptide-based nanoparticles in blood plasma by ...
2015-01-01 [PLoS ONE 10 , e0126136, (2015)] |
|
[Copolymerization of methyl methacrylate with pentafluorophe...
1981-01-01 [Tokyo Ika. Shika. Daigaku. Iyo. Kizai Kenkyusho. Hokoku. 15 , 23-9, (1981)] |
|
Aggregation behavior of amphiphilic p(HPMA)-co-p(LMA) copoly...
2012-12-10 [Biomacromolecules 13(12) , 4065-74, (2012)] |
|
Immobilization of biomolecules to plasma polymerized pentafl...
2010-10-11 [Biomacromolecules 11(10) , 2818-23, (2010)] |
|
Surface reactivity of pulsed-plasma polymerized pentafluorop...
2007-03-27 [Langmuir 23(7) , 3927-31, (2007)] |