High glucose-induced IKK-Hsp-90 interaction contributes to endothelial dysfunction.
Sumathy Mohan, Ryszard Konopinski, Bo Yan, Victoria E Centonze, Mohan Natarajan
文献索引:Am. J. Physiol. Cell Physiol. 296(1) , C182-92, (2009)
全文:HTML全文
摘要
A decline in the bioavailability of nitric oxide (NO) that causes endothelial dysfunction is a hallmark of diabetes. The availability of NO to the vasculature is regulated by endothelial nitric oxide synthase (eNOS) activity and the involvement of heat shock protein-90 (Hsp-90) in the regulation of eNOS activity has been demonstrated. Hsp-90 has been shown to interact with upstream kinases [inhibitor kappaB kinases (IKK)alpha, beta, and gamma] in nonvascular cells. In this study, we have investigated the interaction of Hsp-90-IKKbeta in endothelial cells under conditions of high glucose (HG) as a possible mechanism that diminishes Hsp-90-eNOS interaction, which could contribute to reduced bioavailability of NO. We report for the first time that IKKbeta interacts with Hsp-90, and this interaction is augmented by HG in vascular endothelial cells. HG also augments transcriptional (3.5 +/- 0.65-fold) and translational (1.97 +/- 0.17-fold) expression as well as the catalytic activity of IKKbeta (2.45 +/- 0.4-fold). Both IKKbeta and eNOS could be coimmunoprecipitated with Hsp-90. Inhibition of Hsp-90 with geldanamycin (2 microM) or Radicicol (20 microM) mitigated (0.45 +/- 0.04-fold and 0.93 +/- 0.16-fold, respectively) HG induced-IKKbeta activity (2.5 +/- 0.42-fold). Blocking of IKKbeta expression by IKK inhibitor II (15 microM wedelolactone) or small interferring RNA (siRNA) improved Hsp-90-eNOS interaction and NO production under conditions of HG. These results illuminate a possible mechanism for the declining eNOS activity reported under conditions of HG.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
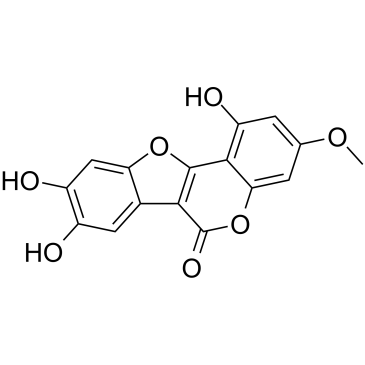 |
蟛蜞菊内脂
CAS:524-12-9 |
C16H10O7 |
|
Understanding the mechanisms controlling Leishmania amazonen...
2014-08-15 [J. Infect. Dis. 210(4) , 656-66, (2014)] |
|
CYLD is a crucial negative regulator of innate immune respon...
2013-11-19 [Cell. Microbiol. 10(11) , 2247-56, (2008)] |
|
Demethylwedelolactone derivatives inhibit invasive growth in...
2012-10-01 [Eur. J. Med. Chem. 56 , 361-7, (2012)] |
|
Wedelolactone, a medicinal plant-derived coumestan, induces ...
2012-12-01 [Int. J. Oncol. 41(6) , 2191-9, (2012)] |
|
Anti-inflammatory gallic Acid and wedelolactone are G protei...
2012-01-01 [Pharmacology 89(3-4) , 211-9, (2012)] |