Investigation of protein structure by means of 19F-NMR. A study of hen egg-white lysozyme.
P Adriaensens, M E Box, H J Martens, E Onkelinx, J Put, J Gelan
文献索引:Eur. J. Biochem. 177(2) , 383-94, (1988)
全文:HTML全文
摘要
A 19F-labeled derivative of hen egg-white lysozyme, in which the six epsilon-amino groups are trifluoroacetylated (LF6), was prepared by reaction of lysozyme with S-ethyltrifluorothioacetate. The reaction mixture was fractionated by cation-exchange chromatography at pH 7.3. A comparison of the circular dichroic spectra and the activity towards Micrococcus lysodeikticus of both LF6 and native lysozyme reveals that the labeling causes no major conformational changes of the polypeptide backbone. Assignment of the six resonances present in the 19F-NMR spectrum of LF6 was accomplished by using a variety of techniques: specific chemical modifications, the effect of the inhibitor (GlcNAc)3, 19F-shift/pH information and relaxation parameters.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
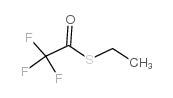 |
三氟硫代乙酸S-乙酯
CAS:383-64-2 |
C4H5F3OS |
|
Fluorinated proteins as potential 19F magnetic resonance ima...
1994-01-01 [Bioconjug. Chem. 5(3) , 257-61, (1994)] |
|
The primary structure of mitochondrial aspartate aminotransf...
1979-01-01 [Ital. J. Biochem. 28(6) , 441-55, (1979)] |
|
Specific cleavage of amino side chains of serine and threoni...
1998-07-01 [Eur. J. Biochem. 255(1) , 162-71, (1998)] |
|
Multiple-sites C-terminal sequencing methods of protein and ...
1998-08-01 [J. Protein Chem. 17(6) , 520-1, (1998)] |
|
A conformational and vibrational study of CF(3)COSCH(2)CH(3)...
2009-12-07 [J. Chem. Phys. 131(21) , 214303, (2009)] |