Mechanism of maltodextrin transport through LamB.
Phillip E Klebba
文献索引:Res. Microbiol. 153(7) , 417-24, (2002)
全文:HTML全文
摘要
The Gram-negative bacterial outer membrane contains several independent, biochemically distinct transport systems for the acquisition of solutes from the environment. Three or more different classes of membrane proteins exist within the porin superfamily, that facilitate the uptake of sugars, amino acids, nucleotides, vitamins and metals. In spite of crystallographic descriptions of these protein transporters over the past decade, the mechanisms by which porins catalyze solute internalization are controversial, and in some cases still obscure. For many years the research of Maurice Hofnung endeavored to explain the transport of maltose and maltodextrins by LamB, also known as maltoporin. In the shadow of recent crystal structures, his work helped outline a different picture of outer membrane transport physiology, that is a tribute to the powerful genetic approaches Maurice pioneered. These data suggest that the principal determinant of maltodextrin recognition by maltoporin derives from the configuration of aromatic amino acids in its surface loops.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
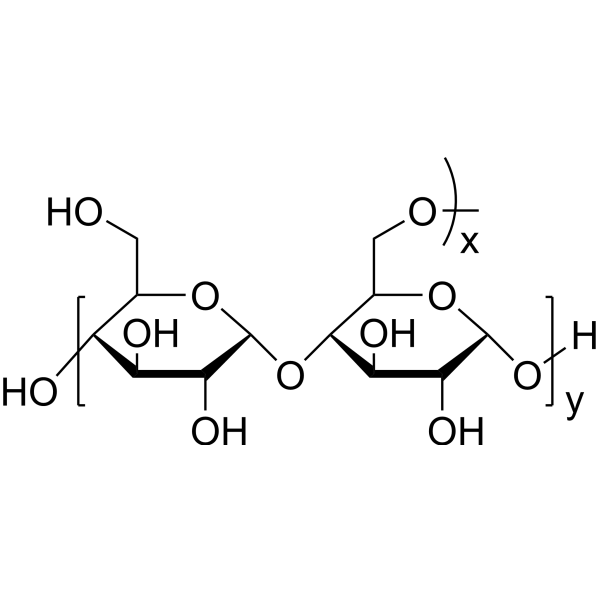 |
麦芽糖糊精
CAS:9050-36-6 |
(C6H10O5)n·xH2O |
|
Identification and characterization of MalA in the maltose/m...
2013-04-01 [J. Bacteriol. 195(8) , 1789-99, (2013)] |
|
Vesicular powder as carrier for doxycycline hydrochloride an...
2014-09-01 [Pharm. Dev. Technol. 19(6) , 755-68, (2014)] |
|
Supramolecular complexes of maltodextrin and furosemide poly...
2013-04-15 [Carbohydr. Polym. 94(1) , 292-300, (2013)] |
|
Synthesis, characterization and toxicological evaluation of ...
2012-01-01 [J. Nanobiotechnology 10 , 47, (2012)] |
|
Prickly pear cactus (Opuntia ficus indica var. saboten) prot...
2012-11-01 [J. Med. Food 15(11) , 968-73, (2012)] |