| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
卵泡刺激素
CAS:9002-68-0 |
|
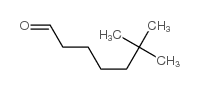 |
尿促性素
CAS:61489-71-2 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
卵泡刺激素
CAS:9002-68-0 |
|
 |
尿促性素
CAS:61489-71-2 |