| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
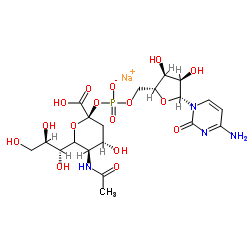 |
唾液
CAS:3063-71-6 |
|
 |
T-细胞生长因子
CAS:94218-72-1 |
|
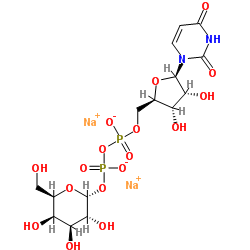 |
UDP-GAL(UDP-半乳糖)二钠盐
CAS:137868-52-1 |
|
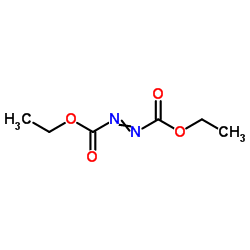 |
偶氮二甲酸二乙酯
CAS:1972-28-7 |