Reversible sialylation: synthesis of cytidine 5'-monophospho-N-acetylneuraminic acid from cytidine 5'-monophosphate with alpha2,3-sialyl O-glycan-, glycolipid-, and macromolecule-based donors yields diverse sialylated products.
E V Chandrasekaran, Jun Xue, Jie Xia, Robert D Locke, Khushi L Matta, Sriram Neelamegham
文献索引:Biochemistry 47(1) , 320-30, (2008)
全文:HTML全文
摘要
Sialyltransferases transfer sialic acid from cytidine 5'-monophospho-N-acetylneuraminic acid (CMP-NeuAc) to an acceptor molecule. Trans-sialidases of parasites transfer alpha2,3-linked sialic acid from one molecule to another without the involvement of CMP-NeuAc. Here we report another type of sialylation, termed reverse sialylation, catalyzed by mammalian sialyltransferase ST3Gal-II. This enzyme synthesizes CMP-NeuAc by transferring NeuAc from the NeuAcalpha2,3Galbeta1,3GalNAcalpha unit of O-glycans, 3-sialyl globo unit of glycolipids, and sialylated macromolecules to 5'-CMP. CMP-NeuAc produced in situ is utilized by the same enzyme to sialylate other O-glycans and by other sialyltransferases such as ST6Gal-I and ST6GalNAc-I, forming alpha2,6-sialylated compounds. ST3Gal-II also catalyzed the conversion of 5'-uridine monophosphate (UMP) to UMP-NeuAc, which was found to be an inactive sialyl donor. Reverse sialylation proceeded without the need for free sialic acid, divalent metal ions, or energy. Direct sialylation with CMP-NeuAc as well as the formation of CMP-NeuAc from 5'-CMP had a wide optimum range (pH 5.2-7.2 and 4.8-6.4, respectively), whereas the entire reaction comprising in situ production of CMP-NeuAc and sialylation of acceptor had a sharp optimum at pH 5.6 (activity level 50% at pH 5.2 and 6.8, 25% at pH 4.8 and 7.2). Several properties distinguish forward/conventional versus reverse sialylation: (i) sodium citrate inhibited forward sialylation but not reverse sialylation; (ii) 5'-CDP, a potent forward sialyltransferase inhibitor, did not inhibit the conversion of 5'-CMP to CMP-NeuAc; and (iii) the mucin core 2 compound 3-O-sulfoGalbeta1,4GlcNAcbeta1,6(Galbeta1,3)GalNAcalpha-O-benzyl, an efficient acceptor for ST3Gal-II, inhibited the conversion of 5'-CMP to CMP-NeuAc. A significant level of reverse sialylation activity is noted in human prostate cancer cell lines LNCaP and PC3. Overall, the study demonstrates that the sialyltransferase reaction is readily reversible in the case of ST3Gal-II and can be exploited for the enzymatic synthesis of diverse sialyl products.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
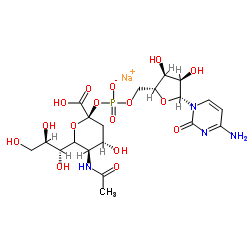 |
唾液
CAS:3063-71-6 |
C20H30N4NaO16P |
|
Glycosylation of immunoglobulin G determines osteoclast diff...
2015-01-01 [Nat. Commun. 6 , 6651, (2015)] |
|
In vitro glycoengineering of IgG1 and its effect on Fc recep...
2016-08-01 [PLoS ONE 10 , e0134949, (2015)] |
|
A broadly-protective vaccine against meningococcal disease i...
2014-05-01 [Vaccine 32(23) , 2688-95, (2014)] |
|
Crystal structures of sialyltransferase from Photobacterium ...
2014-12-20 [FEBS Lett. 588(24) , 4720-9, (2014)] |
|
A native outer membrane vesicle vaccine confers protection a...
2015-03-10 [Vaccine 33(11) , 1317-23, (2015)] |